Molecular mechanisms of regulation of fast-inactivating voltage-dependent transient outward K+ current in mouse heart by cell volume changes
- PMID: 16081489
- PMCID: PMC1474744
- DOI: 10.1113/jphysiol.2005.091264
Molecular mechanisms of regulation of fast-inactivating voltage-dependent transient outward K+ current in mouse heart by cell volume changes
Abstract
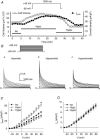
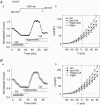
The K(v)4.2/4.3 channels are the primary subunits that contribute to the fast-inactivating, voltage-dependent transient outward K(+) current (I(to,fast)) in the heart. I(to,fast) is the critical determinant of the early repolarization of the cardiac action potential and plays an important role in the adaptive remodelling of cardiac myocytes, which usually causes cell volume changes, during myocardial ischaemia, hypertrophy and heart failure. It is not known, however, whether I(to,fast) is regulated by cell volume changes. In this study we investigated the molecular mechanism for cell volume regulation of I(to,fast) in native mouse left ventricular myocytes. Hyposmotic cell swelling caused a marked increase in densities of the peak I(to,fast) and a significant shortening in phase 1 repolarization of the action potential duration. The voltage-dependent gating properties of I(to,fast) were, however, not altered by changes in cell volume. In the presence of either protein kinase C (PKC) activator (12,13-dibutyrate) or phosphatase inhibitors (calyculin A and okadaic acid), hyposmotic cell swelling failed to further up-regulate I(to,fast). When expressed in NIH/3T3 cells, both K(v)4.2 and K(v)4.3 channels were also strongly regulated by cell volume in the same voltage-independent but PKC- and phosphatase-dependent manner as seen in I(to,fast) in the native cardiac myocytes. We conclude that K(v)4.2/4.3 channels in the heart are regulated by cell volume through a phosphorylation/dephosphorylation pathway mediated by PKC and serine/threonine phosphatase(s). These findings suggest a novel role of K(v)4.2/4.3 channels in the adaptive electrical and structural remodelling of cardiac myocytes in response to myocardial hypertrophy, ischaemia and reperfusion.
Figures








Similar articles
-
Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes.Circ Res. 2005 Mar 4;96(4):451-8. doi: 10.1161/01.RES.0000156890.25876.63. Epub 2005 Jan 20. Circ Res. 2005. PMID: 15662035
-
Central role of PKCα in isoenzyme-selective regulation of cardiac transient outward current Ito and Kv4.3 channels.J Mol Cell Cardiol. 2011 Nov;51(5):722-9. doi: 10.1016/j.yjmcc.2011.07.012. Epub 2011 Jul 22. J Mol Cell Cardiol. 2011. PMID: 21803046
-
Interaction of DPP10a with Kv4.3 channel complex results in a sustained current component of human transient outward current Ito.Basic Res Cardiol. 2015 Mar;110(2):5. doi: 10.1007/s00395-014-0457-2. Epub 2015 Jan 20. Basic Res Cardiol. 2015. PMID: 25600224
-
Function and regulation of serine/threonine phosphatases in the healthy and diseased heart.J Mol Cell Cardiol. 2013 Nov;64:90-8. doi: 10.1016/j.yjmcc.2013.09.006. Epub 2013 Sep 16. J Mol Cell Cardiol. 2013. PMID: 24051368 Review.
-
Transient outward potassium current, 'Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms.J Physiol. 2005 Nov 15;569(Pt 1):7-39. doi: 10.1113/jphysiol.2005.086223. Epub 2005 Apr 14. J Physiol. 2005. PMID: 15831535 Free PMC article. Review.
Cited by
-
Regulation of cell volume and water transport--an old fundamental role of the renin angiotensin aldosterone system components at the cellular level.Peptides. 2014 Aug;58:74-7. doi: 10.1016/j.peptides.2014.06.003. Epub 2014 Jun 16. Peptides. 2014. PMID: 24945466 Free PMC article. Review.
-
Heart-specific overexpression of the human short CLC-3 chloride channel isoform limits myocardial ischemia-induced ERP and QT prolongation.Int J Cardiol. 2016 Jul 1;214:218-24. doi: 10.1016/j.ijcard.2016.03.191. Epub 2016 Mar 30. Int J Cardiol. 2016. PMID: 27064645 Free PMC article.
-
Resolution of hyposmotic stress in isolated mouse ventricular myocytes causes sealing of t-tubules.Exp Physiol. 2013 Jul;98(7):1164-77. doi: 10.1113/expphysiol.2013.072470. Epub 2013 Apr 12. Exp Physiol. 2013. PMID: 23585327 Free PMC article.
-
Double the keys, double the control: coupled phosphorylation sites provide novel molecular targets for precise control of ion channel function. Focus on "Differential regulation of a CLC anion channel by SPAK kinase ortholog-mediated multisite phosphorylation".Am J Physiol Cell Physiol. 2012 Jun 15;302(12):C1699-701. doi: 10.1152/ajpcell.00107.2012. Epub 2012 Apr 11. Am J Physiol Cell Physiol. 2012. PMID: 22496246 Free PMC article. No abstract available.
References
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998;83:560–567. - PubMed
-
- Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. - PubMed
-
- Befroy DE, Powell T, Radda GK, Clarke K. Osmotic shock: modulation of contractile function, pHi, and ischemic damage in perfused guinea pig heart. Am J Physiol. 1999;276:H1236–H1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

