Genetic reprogramming of tumor cells by zinc finger transcription factors
- PMID: 16081541
- PMCID: PMC1187960
- DOI: 10.1073/pnas.0501162102
Genetic reprogramming of tumor cells by zinc finger transcription factors
Abstract
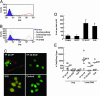
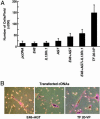
Cancer arises by the accumulation of genetic alterations in DNA leading to aberrant gene transcription. Expression-profiling studies have correlated genomewide expression signatures with malignancy. However, functional analysis elucidating the contribution and synergy of genes in specific cancer cell phenotypes remains a formidable obstacle. Herein, we describe an alternative genetic approach for identification of genes involved in tumor progression by using a library of zinc finger artificial transcription factors (ATFs) and functional screening of tumor cells as a source of genetic plasticity and clonal selection. We isolated a six-zinc finger transcriptional activator (TF 20-VP, TF 20 containing the VP64 activator domain) that acts to reprogram a drug-sensitive, poorly invasive, and nonmetastatic cell line into a cell line with a drug-resistant, highly invasive, and metastatic phenotype. Differential expression profiles of cells expressing TF 20-VP followed by functional studies, both in vitro and in animal models, revealed that invasion and metastasis requires co-regulation of multiple target genes. Significantly, the E48 antigen, associated with poor metastasis-free survival in head and neck cancer, was identified as one specific target of TF 20-VP. We have shown phenotypic modulation of tumor cell behavior by E48 expression, including enhanced cell migration in vitro and tumor cell dissemination in vivo. This study demonstrates the use of ATFs to identify the group of genes that cooperate during tumor progression. By co-regulating multiple targets, ATFs can be used as master genetic switches to reprogram and modulate complex neoplastic phenotypes.
Figures


References
-
- Ramaswamy, S., Ross, K. N., Lander, E. S. & Golub, T. R. (2003) Nat. Genet. 33, 49–87. - PubMed
-
- Segal, E., Friedman, N. & Regev, A. (2004) Nat. Genet. 36, 1090–1098. - PubMed
-
- Beneklli, M., Baer, M. R., Baumann, H. & Wetzier, M. (2003) Blood 101, 2940–2951. - PubMed
-
- Kang, Y. & Massague, J. E. (2004) Cell 118, 277–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

