Ionic interactions at both inter-ring contact sites of GroEL are involved in transmission of the allosteric signal: a time-resolved infrared difference study
- PMID: 16081650
- PMCID: PMC2253480
- DOI: 10.1110/ps.051469605
Ionic interactions at both inter-ring contact sites of GroEL are involved in transmission of the allosteric signal: a time-resolved infrared difference study
Abstract
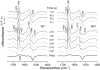
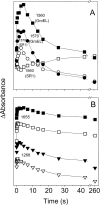
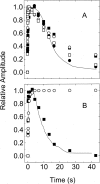
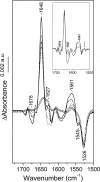
The biological activity of the double-ring chaperonin GroEL is regulated by complex allosteric interactions, which include positive intra-ring and negative inter-ring cooperativity. To further characterize inter-ring communication, the nucleotide-induced absorbance changes in the vibrational spectrum of the chaperonin GroEL, of two single-point mutants suppressing one inter-ring ionic contact (E461K and E434K) and of a single-ring version of this protein, were investigated by time-resolved infrared difference spectroscopy. Interaction of the nucleotide with the proteins was triggered by its photochemical release from a biologically inactive caged precursor [P3-1-(2-nitro) phenylethyl nucleotide]. The results indicate that (1) ATP binding to the protein induces a conformational change that affects concomitantly both intra-ring and inter-ring communication, and (2) the experimental absorbance changes are sensitive to the double-ring structure of the protein. The characterization of the single-point, inter-ring mutants demonstrates that ionic interactions at both contact sites are involved in the transmission of the allosteric signal. However, both mutations have different effects on the inter-ring interface. While that of E461K still retains ionic contacts sensitive to ATP binding, E434K shows spectroscopic features similar to those of the single-ring version of the protein, therefore suggesting that electrostatic interactions at these contact sites contribute differently to the stability of the inter-ring interface.
Figures
Similar articles
-
A kinetic analysis of the nucleotide-induced allosteric transitions in a single-ring mutant of GroEL.J Mol Biol. 2004 May 14;338(5):969-77. doi: 10.1016/j.jmb.2004.03.010. J Mol Biol. 2004. PMID: 15111060
-
Conversion of the allosteric transition of GroEL from concerted to sequential by the single mutation Asp-155 -> Ala.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13797-802. doi: 10.1073/pnas.2333925100. Epub 2003 Nov 13. Proc Natl Acad Sci U S A. 2003. PMID: 14615587 Free PMC article.
-
GroEL stability and function. Contribution of the ionic interactions at the inter-ring contact sites.J Biol Chem. 2003 Aug 22;278(34):32083-90. doi: 10.1074/jbc.M303958200. Epub 2003 Jun 9. J Biol Chem. 2003. PMID: 12796493
-
Review: allostery in chaperonins.J Struct Biol. 2001 Aug;135(2):104-14. doi: 10.1006/jsbi.2001.4377. J Struct Biol. 2001. PMID: 11580260 Review.
-
New structures of allosteric proteins revealing remarkable conformational changes.Curr Opin Struct Biol. 1996 Dec;6(6):824-9. doi: 10.1016/s0959-440x(96)80013-3. Curr Opin Struct Biol. 1996. PMID: 8994883 Review.
Cited by
-
Macromolecule-assisted de novo protein folding.Int J Mol Sci. 2012;13(8):10368-10386. doi: 10.3390/ijms130810368. Epub 2012 Aug 20. Int J Mol Sci. 2012. PMID: 22949867 Free PMC article. Review.
-
Inter-ring communication allows the GroEL chaperonin complex to distinguish between different substrates.Protein Sci. 2007 May;16(5):956-65. doi: 10.1110/ps.062713607. Protein Sci. 2007. PMID: 17456746 Free PMC article.
-
Structural basis for active single and double ring complexes in human mitochondrial Hsp60-Hsp10 chaperonin.Nat Commun. 2020 Apr 21;11(1):1916. doi: 10.1038/s41467-020-15698-8. Nat Commun. 2020. PMID: 32317635 Free PMC article.
References
-
- Amir, A. and Horovitz, A. 2004. Kinetic analysis of ATP-dependent interring communication in GroEL. J. Mol. Biol. 338 979–988. - PubMed
-
- Barth, A. 2000. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 74 141–173. - PubMed
-
- Barth, A. and Zscherp, C. 2000. Substrate binding and enzyme function investigated by infrared spectroscopy. FEBS Lett. 477 151–156. - PubMed
-
- ———. 2002. What vibrations tell us about proteins. Q. Rev. Biophys. 35 369–430. - PubMed
-
- Barth, A., Mäntele, W., and Kreutz, W. 1991. Infrared spectroscopic signals arising from ligand binding and conformational changes in the catalytic cycle of sarcoplasmic reticulum calcium ATPase. Biochim. Biophys. Acta 1057 115–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

