Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural
- PMID: 16081669
- PMCID: PMC2680124
- DOI: 10.1634/stemcells.2004-0284
Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural
Abstract
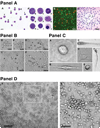
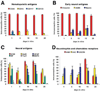
The mononuclear fraction from human umbilical cord blood (HUCB) contains a significant number of stem/progenitor cells that in theory could be come any cell in the body, including neurons. Taking into consideration that transdifferentiation would be a very rare event and also knowing that overlapping genetic programs for hematopoiesis and neuropoiesis exist, we undertook a characterization of the HUCB mononuclear fraction, including analysis of cellular subpopulations and their morphology, cell viability, proliferation, and expression of neural and hematopoietic antigens. Two cell populations were apparent-adherent and floating fractions. The adherent fraction was mainly lymphocytes (~53%) expressing hematopoietic antigens. Upon replate, the floating population had many cells that expressed stem cell antigens. More of the cells in this subfraction expressed neural proteins. Neurotrophin receptors trkB and trkC were present in both cell fractions, although expression was higher in the floating fraction. Our initial characterization suggests that a subpopulation of cells exists within the HUCB mononuclear fraction that seems to have the potential to become neural cells, which could then be used in the development of cell-based therapies for brain injuries and diseases.
Figures


References
-
- de Medeiros CR, Silva LM, Pasquini R. Unrelated cord blood transplantation in a Fanconi anemia patient using fludarabine-based conditioning. Bone Marrow Transplant. 2001;28:110–112. - PubMed
-
- Ooi J, Iseki T, Nagayama H, et al. Unrelated cord blood transplantation for adult patients with myelodysplastic syndrome-related secondary acute myeloid leukaemia. Br J Haematol. 2001;114:834–836. - PubMed
-
- Tezuka K, Nakayama H, Honda K, et al. Treatment of a child with myeloid/NK cell precursor acute leukemia with L-asparaginase and unrelated cord blood transplantation. Int J Hematol. 2002;75:201–206. - PubMed
-
- Locatelli F, Rocha V, Reed W, et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. - PubMed
-
- Krisiukeniene A, Sitkauskiene B, Sakalauskas R. Wiskott-Aldrich syndrome: the possibilities of diagnosis and treatment [in Lithuanian] Medicina (Kaunas) 2003;39:211–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

