Evaluation of the VACUTAINER PPT Plasma Preparation Tube for use with the Bayer VERSANT assay for quantification of human immunodeficiency virus type 1 RNA
- PMID: 16081908
- PMCID: PMC1233973
- DOI: 10.1128/JCM.43.8.3769-3771.2005
Evaluation of the VACUTAINER PPT Plasma Preparation Tube for use with the Bayer VERSANT assay for quantification of human immunodeficiency virus type 1 RNA
Abstract
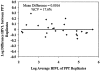
Separation and storage of plasma within 2 h of phlebotomy is required for the VACUTAINER PPT Plasma Preparation Tube (PPT) versus 4 h for the predecessor VACUTAINER EDTA tube for human immunodeficiency virus type 1 (HIV-1) viral load (HIVL) testing by the VERSANT HIV-1 RNA 3.0 assay (branched DNA). The 2-h limit for PPT imposes time constraints for handling and transporting to the testing laboratory. This study compares HIVL reproducibility from matched blood in EDTA tubes and PPTs and between PPT pairs following processing within 4 h of phlebotomy, stability of plasma HIV-1 RNA at 24- and 72-h room temperature storage in the tube, and comparative labor and supply requirements. Blood from 159 patients was collected in paired tubes (EDTA/PPT or PPT/PPT): 86 paired EDTA tubes and PPTs were processed 4 h following phlebotomy and their HIVLs were compared, 42 paired PPT/PPT pairs were analyzed for intertube HIVL reproducibility, and 31 PPT/PPT pairs were analyzed for HIV-1 RNA stability by HIVL. Labor and supply requirements were compared between PPT and EDTA tubes. PPTs produce results equivalent to standard EDTA tube results when processed 4 h after phlebotomy. PPT intertube analyte results are reproducible. An average decrease of 13% and 37% in HIVL was observed in PPT plasma after 24 and 72 h of room temperature storage, respectively; thus, plasma can be stored at room temperature up to 24 h in the original tube. PPTs offer labor and supply savings over EDTA tubes.
Figures

References
-
- Bland, J. M., and D. G. Altman. 1995. Comparing two methods of clinical measurement: a personal history. Int. J. Epidemiol. 24(Suppl. 1):S7-S14. - PubMed
-
- Collins, M. L., B. Irvine, D. Tyner, E. Fine, C. Zayati, C. Chang, T. Horn, D. Ahle, J. Detmer, L. P. Shen, J. Kolberg, S. Bushnell, M. S. Urdea, and D. D. Ho. 1997. A branched DNA signal amplification assay for quantification of nucleic acid targets below 100 molecules/ml. Nucleic Acids Res. 25:2979-2984. - PMC - PubMed
-
- Gleaves, C. A., J. Welle, M. Campbell, T. Elbeik, V. Ng, P. E. Taylor, K. Kuramoto, S. Aceituno, E. Lewalski, B. Joppa, L. Sawyer, C. Schaper, D. McNairn, and T. Quinn. 2002. Multicenter evaluation of the Bayer VERSANT HIV-1 RNA 3.0 assay: analytical and clinical performance. J. Clin. Virol. 25:205-216. - PubMed
-
- Holodniy, M., L. Mole, B. Yen-Lieberman, D. Margolis, C. Starkey, R. Carroll, T. Spahlinger, J. Todd, and J. B. Jackson. 1995. Comparative stabilities of quantitative human immunodeficiency virus RNA in plasma from samples collected in VACUTAINER CPT, VACUTAINER PPT, and standard VACUTAINER tubes. J. Clin. Microbiol. 33:1562-1566. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

