The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases
- PMID: 16083423
- PMCID: PMC1237134
- DOI: 10.1042/BJ20051180
The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases
Abstract
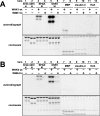
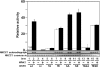
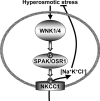
Mutations in the human genes encoding WNK1 [with no K (lysine) protein kinase-1] and the related protein kinase WNK4 are the cause of Gordon's hypertension syndrome. Little is known about the molecular mechanism by which WNK isoforms regulate cellular processes. We immunoprecipitated WNK1 from extracts of rat testis and found that it was specifically associated with a protein kinase of the STE20 family termed 'STE20/SPS1-related proline/alanine-rich kinase' (SPAK). We demonstrated that WNK1 and WNK4 both interacted with SPAK as well as a closely related kinase, termed 'oxidative stress response kinase-1' (OSR1). Wildtype (wt) but not catalytically inactive WNK1 and WNK4 phosphorylated SPAK and OSR1 to a much greater extent than with other substrates utilized previously, such as myelin basic protein and claudin-4. Phosphorylation by WNK1 or WNK4 markedly increased SPAK and OSR1 activity. Phosphopeptide mapping studies demonstrated that WNK1 phosphorylated kinase-inactive SPAK and OSR1 at an equivalent residue located within the T-loop of the catalytic domain (Thr233 in SPAK, Thr185 in OSR1) and a serine residue located within a C-terminal non-catalytic region (Ser373 in SPAK, Ser325 in OSR1). Mutation of Thr185 to alanine prevented the activation of OSR1 by WNK1, whereas mutation of Thr185 to glutamic acid (to mimic phosphorylation) increased the basal activity of OSR1 over 20-fold and prevented further activation by WNK1. Mutation of Ser325 in OSR1 to alanine or glutamic acid did not affect the basal activity of OSR1 or its ability to be activated by WNK1. These findings suggest that WNK isoforms operate as protein kinases that activate SPAK and OSR1 by phosphorylating the T-loops of these enzymes, resulting in their activation. Our analysis also describes the first facile assay that can be employed to quantitatively assess WNK1 and WNK4 activity.
Figures





Comment in
-
WNK lies upstream of kinases involved in regulation of ion transporters.Biochem J. 2005 Oct 1;391(Pt 1):e1-3. doi: 10.1042/BJ20051345. Biochem J. 2005. PMID: 16173916 Free PMC article.
References
-
- Xu B., English J. M., Wilsbacher J. L., Stippec S., Goldsmith E. J., Cobb M. H. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. - PubMed
-
- Verissimo F., Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. - PubMed
-
- Wilson F. H., Disse-Nicodeme S., Choate K. A., Ishikawa K., Nelson-Williams C., Desitter I., Gunel M., Milford D. V., Lipkin G. W., Achard J. M., et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
-
- Zambrowicz B. P., Abuin A., Ramirez-Solis R., Richter L. J., Piggott J., BeltrandelRio H., Buxton E. C., Edwards J., Finch R. A., Friddle C. J., et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14109–14114. - PMC - PubMed
-
- Kamide K., Takiuchi S., Tanaka C., Miwa Y., Yoshii M., Horio T., Mannami T., Kokubo Y., Tomoike H., Kawano Y., Miyata T. Three novel missense mutations of WNK4, a kinase mutated in inherited hypertension, in Japanese hypertensives: implication of clinical phenotypes. Am. J. Hypertens. 2004;17:446–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

