Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase
- PMID: 16083424
- PMCID: PMC1317667
- DOI: 10.1042/BJ20051102
Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase
Abstract
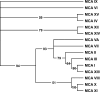
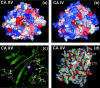
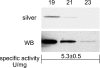
The main function of CAs (carbonic anhydrases) is to participate in the regulation of acid-base balance. Although 12 active isoenzymes of this family had already been described, analyses of genomic databases suggested that there still exists another isoenzyme, CA XV. Sequence analyses were performed to identify those species that are likely to have an active form of this enzyme. Eight species had genomic sequences encoding CA XV, in which all the amino acid residues critical for CA activity are present. However, based on the sequence data, it was apparent that CA XV has become a non-processed pseudogene in humans and chimpanzees. RT-PCR (reverse transcriptase PCR) confirmed that humans do not express CA XV. In contrast, RT-PCR and in situ hybridization performed in mice showed positive expression in the kidney, brain and testis. A prediction of the mouse CA XV structure was performed. Phylogenetic analysis showed that mouse CA XV is related to CA IV. Therefore both of these enzymes were expressed in COS-7 cells and studied in parallel experiments. The results showed that CA XV shares several properties with CA IV, i.e. it is a glycosylated glycosylphosphatidylinositol-anchored membrane protein, and it binds CA inhibitor. The catalytic activity of CA XV is low, and the correct formation of disulphide bridges is important for the activity. Both specific and non-specific chaperones increase the production of active enzyme. The results suggest that CA XV is the first member of the alpha-CA gene family that is expressed in several species, but not in humans and chimpanzees.
Figures

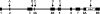
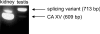

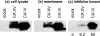

References
-
- Lindskog S., Silverman D. N. The catalytic mechanism of mammalian carbonic anhydrases. In: Chegwidden W. R., Carter N. D., Edwards Y. H., editors. The Carbonic Anhydrases: New Horizons. Basel: Birkhäuser Verlag; 2000. pp. 175–195. - PubMed
-
- Sly W. S., Hu P. Y. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu. Rev. Biochem. 1995;64:375–401. - PubMed
-
- Hewett-Emmett D. Evolution and distribution of the carbonic anhydrase gene families. In: Chegwidden W. R., Carter N. D., Edwards Y. H., editors. The Carbonic Anhydrases: New Horizons. Basel: Birhkhäuser Verlag; 2000. pp. 29–76. - PubMed
-
- Lehtonen J., Shen B., Vihinen M., Casini A., Scozzafava A., Supuran C. T., Parkkila A. K., Saarnio J., Kivela A. J., Waheed A., et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isoenzyme family. J. Biol. Chem. 2004;279:2719–2727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

