The pore structure of the closed RyR1 channel
- PMID: 16084392
- PMCID: PMC2983469
- DOI: 10.1016/j.str.2005.06.005
The pore structure of the closed RyR1 channel
Abstract
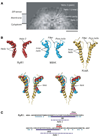
Using single particle electron cryomicroscopy, several helices in the membrane-spanning region of RyR1, including an inner transmembrane helix, a short pore helix, and a helix parallel to the membrane on the cytoplasmic side, have been clearly resolved. Our model places a highly conserved glycine (G4934) at the hinge position of the bent inner helix and two rings of negative charges at the luminal and cytoplasmic mouths of the pore. The kinked inner helix closely resembles the inner helix of the open MthK channel, suggesting that kinking alone does not open RyR1, as proposed for K+ channels.
Figures






Comment in
-
Bend to open?Structure. 2005 Aug;13(8):1094-5. doi: 10.1016/j.str.2005.07.004. Structure. 2005. PMID: 16084381 No abstract available.
References
-
- Baker ML, Jiang W, Chiu W. Analysis of intermediate resolution structures. Biophys. J. 2005;88:23.
-
- Booth CR, Jiang W, Baker ML, Hong Zhou Z, Ludtke SJ, Chiu W. A 9 Å single particle reconstruction from CCD coucaptured images on a 200 kV electron cryomicroscope. J. Struct. Biol. 2004;147:116–127. - PubMed
-
- Böttcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. - PubMed
-
- Callaway C, Seryshev A, Wang JP, Slavik KJ, Needleman DH, Cantu C, 3rd, Wu Y, Jayaraman T, Marks AR, Hamilton SL. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+ release channel. J. Biol. Chem. 1994;269:15876–15884. - PubMed
-
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

