Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction
- PMID: 16085713
- PMCID: PMC1187969
- DOI: 10.1073/pnas.0502488102
Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction
Abstract
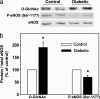
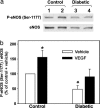
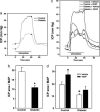
Impaired endothelial nitric oxide synthase (eNOS) function is associated with erectile dysfunction in diabetes mellitus, but the exact molecular basis for the eNOS defect in the diabetic penis remains unclear. We investigated whether hyperglycemia increases O-GlcNAc modification of eNOS in the penis, preventing phosphorylation at the primary positive regulatory site on the enzyme and hampering mechanisms of the erectile response. Type I diabetes mellitus was induced in male rats by alloxan (140 mg/kg, i.p.). After 5 wk, the diabetic rat penis exhibited increased O-GlcNAc modification of eNOS and decreased eNOS phosphorylation at Ser-1177 at baseline compared with the control rat penis; eNOS phosphorylation at Thr-495, Ser-615, and Ser-633 was not affected. In addition, eNOS phosphorylation at Ser-1177 was impaired in the diabetic rat penis in response to penile blood flow (shear stress) elicited by electrical stimulation of the cavernous nerve (ES) and to recombinant human VEGF165. Phosphorylation of Akt, a mediator of shear stress-induced eNOS phosphorylation at Ser-1177, was decreased in the diabetic penis at baseline, but it was restored by ES. Erectile response to shear stress elicited by ES and to VEGF was decreased in diabetic compared with control rats. This work demonstrates that eNOS inactivation occurs in the diabetic penis by a glycosylation mechanism specifically at Ser-1177, by which the enzyme is rendered incapable of activation by fluid shear stress stimuli and VEGF signaling. In vivo penile erection paradigm supports the physiologic relevance of O-GlcNAc modification in vascular disorders associated with diabetes.
Figures
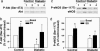

References
-
- Musicki, B., Palese, M. A., Crone, J. K. & Burnett, A. L. (2004) Biol. Reprod. 70, 282-289. - PubMed
-
- Harris, M. B., Ju, H., Venema, V. J., Liang, H., Zou. R., Michell, B. J., Chen, Z. P., Kemp, B. E. & Venema, R. C. (2001) J. Biol. Chem. 276, 16587-16591. - PubMed
-
- Michell, B. J., Chen, Z., Tiganis, T., Stapleton, D., Katsis, F., Power, D. A., Sim, A. T. & Kemp, B. E. (2001) J. Biol. Chem. 276, 17625-17628. - PubMed
-
- Fleming, I., Fisslthaler, B., Dimmeler, S., Kemp, B. E. & Busse, R. (2001) Circ. Res. 88, E68-E75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

