1,2-diacyl-phosphatidylcholine flip-flop measured directly by sum-frequency vibrational spectroscopy
- PMID: 16085770
- PMCID: PMC1366751
- DOI: 10.1529/biophysj.105.065672
1,2-diacyl-phosphatidylcholine flip-flop measured directly by sum-frequency vibrational spectroscopy
Abstract
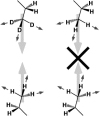
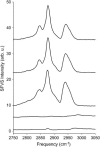
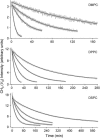
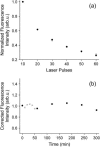
Sum-frequency vibrational spectroscopy (SFVS) is used to measure the intrinsic rate of lipid flip-flop for 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) in planar-supported lipid bilayers (PSs). Asymmetric PSLBs were prepared using the Langmuir-Blodgett/Langmuir-Schaefer method by placing a perdeuterated lipid analog in one leaflet of the PSLB. SFVS was used to directly measure the asymmetric distribution of the native lipid within the membrane by measuring the decay in the CH3 v(s) intensity at 2875 cm(-1) with time and as a function of temperature. An average activation energy of 220 kJ/mol for the translocation of DMPC, DPPC, and DSPC was determined. A decrease in alkyl chain length resulted in a substantial increase in the rate of flip-flop manifested as an increase in the Arrhenius preexponential factor. The effect of lipid labeling was investigated by measuring the exchange of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-n,n-Dimethyl-n-(2',2',6',6'-tetramethyl-4'-piperidyl) (TEMPO-DPPC). The rate of TEMPO-DPPC flip-flop was an order-of-magnitude slower compared to DPPC. An activation energy of 79 kJ/mol was measured which is comparable to that previously measured by electron spin resonance. The results of this study illustrate how SFVS can be used to directly measure lipid flip-flop without the need for a fluorescent or spin-labeled lipid probe, which can significantly alter the rate of lipid translocation.
Figures


Similar articles
-
The effect of cholesterol on the intrinsic rate of lipid flip-flop as measured by sum-frequency vibrational spectroscopy.Faraday Discuss. 2013;161:45-61; discussion 113-50. doi: 10.1039/c2fd20083j. Faraday Discuss. 2013. PMID: 23805737
-
Issues with lipid probes in flip-flop measurements: A comparative study using sum-frequency vibrational spectroscopy and second-harmonic generation.J Chem Phys. 2024 Aug 28;161(8):085104. doi: 10.1063/5.0226075. J Chem Phys. 2024. PMID: 39185850
-
Free energy and entropy of activation for phospholipid flip-flop in planar supported lipid bilayers.J Phys Chem B. 2010 Feb 11;114(5):1903-14. doi: 10.1021/jp909134g. J Phys Chem B. 2010. PMID: 20073520
-
Sum-frequency vibrational spectroscopy, a tutorial: Applications for the study of lipid membrane structure and dynamics.Biointerphases. 2024 May 1;19(3):031201. doi: 10.1116/6.0003594. Biointerphases. 2024. PMID: 38738942 Review.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Atomistic simulations of pore formation and closure in lipid bilayers.Biophys J. 2014 Jan 7;106(1):210-9. doi: 10.1016/j.bpj.2013.11.4486. Biophys J. 2014. PMID: 24411253 Free PMC article.
-
Observing a model ion channel gating action in model cell membranes in real time in situ: membrane potential change induced alamethicin orientation change.J Am Chem Soc. 2012 Apr 11;134(14):6237-43. doi: 10.1021/ja2110784. Epub 2012 Apr 3. J Am Chem Soc. 2012. PMID: 22420296 Free PMC article.
-
Facile lipid flip-flop in a phospholipid bilayer induced by gramicidin A measured by sum-frequency vibrational spectroscopy.Biophys J. 2007 Jan 1;92(1):L01-3. doi: 10.1529/biophysj.106.096057. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071658 Free PMC article.
-
Fluorescence lifetime tuning--a novel approach to study flip-flop kinetics in supported phospholipid bilayers.J Fluoresc. 2010 Mar;20(2):563-9. doi: 10.1007/s10895-009-0581-9. Epub 2009 Dec 29. J Fluoresc. 2010. PMID: 20039107
-
Second Harmonic Generation Spectroscopy of Membrane Probe Dynamics in Gram-Positive Bacteria.Biophys J. 2019 Oct 15;117(8):1419-1428. doi: 10.1016/j.bpj.2019.09.014. Epub 2019 Sep 18. Biophys J. 2019. PMID: 31586521 Free PMC article.
References
-
- Kol, M. A., B. de Kruijff, and A. I. P. M. de Kroon. 2002. Phospholipid flip-flop in biogenic membranes: what is needed to connect opposite sides. Semin. Cell Dev. Biol. 13:163–170. - PubMed
-
- Buton, X., G. Morrot, P. Fellmann, and M. Seigneuret. 1996. Ultra-fast glycerophospholipid selective transbilayer motion mediated by a protein in the endoplasmic reticulum membrane. J. Biol. Chem. 271:6651–6658. - PubMed
-
- Herrmann, A., A. Zachowski, and P. F. Devaux. 1990. Protein mediated phospholipid translocation in the endoplasmic reticulum with a low lipid specificity. Biochemistry. 29:2023–2027. - PubMed
-
- Martin, O. C., and R. E. Pagano. 1987. Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J. Biol. Chem. 262:5890–5898. - PubMed
-
- Eytan, G. D., R. Regev, G. Oren, and Y. G. Assaraf. 1996. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J. Biol. Chem. 271:12897–12902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

