The hydroxylamine reaction of sensory rhodopsin II: light-induced conformational alterations with C13=C14 nonisomerizable pigment
- PMID: 16085771
- PMCID: PMC1366761
- DOI: 10.1529/biophysj.105.065631
The hydroxylamine reaction of sensory rhodopsin II: light-induced conformational alterations with C13=C14 nonisomerizable pigment
Abstract
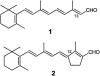
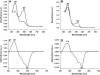
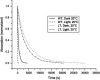
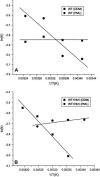
Sensory rhodopsin II, a repellent phototaxis receptor from Natronomonas (Natronobacterium) pharaonis (NpSRII), forms a complex with its cognate transducer (NpHtrII). In micelles the two proteins form a 1:1 heterodimer, whereas in membranes they assemble to a 2:2 complex. Similarly to other retinal proteins, sensory rhodopsin II undergoes a bleaching reaction with hydroxylamine in the dark which is markedly catalyzed by light. The reaction involves cleavage of the protonated Schiff base bond which covalently connects the retinal chromophore to the protein. The light acceleration reflects protein conformation alterations, at least in the retinal binding site, and thus allows for detection of these changes in various conditions. In this work we have followed the hydroxylamine reaction at different temperatures with and without the cognate transducer. We have found that light irradiation reduces the activation energy of the hydroxylamine reaction as well as the frequency factor. A similar effect was found previously for bacteriorhodopsin. The interaction with the transducer altered the light effect both in detergent and membranes. The transducer interaction decreased the apparent light effect on the energy of activation and the frequency factor in detergent but increased it in membranes. In addition, we have employed an artificial pigment derived from a retinal analog in which the critical C13=C14 double bond is locked by a rigid ring structure preventing its isomerization. We have observed light enhancement of the reaction rate and reduction of the energy of activation as well as the frequency factor, despite the fact that this pigment does not experience C13=C14 double bond isomerization. It is suggested that retinal excited state polarization caused by light absorption of the "locked" pigment polarizes the protein and triggers relatively long-lived protein conformational alterations.
Figures

References
-
- Sasaki, J., and J. L. Spudich. 2000. Proton transport by sensory rhodopsins and its modulation by transducer-binding. Biochim. Biophys. Acta. 1460:230–239. - PubMed
-
- Haupts, U., J. Tittor, and D. Oesterhelt. 1999. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 28:367–399. - PubMed
-
- Lanyi, J. K. 2000. Molecular mechanism of ion transport in bacteriorhodopsin: insights from crystallographic, spectroscopic, kinetic, and mutational studies. J. Phys. Chem. B. 104:11441–11448.
-
- Klare, J., V. Gordeliy, J. Labahn, G. Büldt, H. J. Steinhoff, and M. Engelhard. 2004. The archaeal sensory rhodopsin II/transducer complex: a model for transmembrane signal transfer. FEBS Lett. 564:219–224. - PubMed
-
- Spudich, J. L. 1998. Variations on a molecular switch: transport and sensory signalling by archaeal rhodopsins. Mol. Microbiol. 28:1051–1058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

