Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0
- PMID: 16085802
- PMCID: PMC1183334
- DOI: 10.1128/AEM.71.8.4191-4198.2005
Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0
Abstract
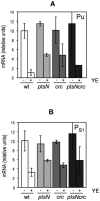
Toluene degradation in Pseudomonas putida KT2440 pWW0 plasmid is subjected to catabolite repression. Pu and P(S1) promoters of the pWW0 TOL plasmid are down-regulated in vivo during exponential growth in rich medium. In cells growing on minimal medium, yeast extract (YE) addition mimics exponential-phase rich medium repression of these promoters. We have constructed and tested mutants in a series of global regulators described in Pseudomonas. We describe that a mutant in crc (catabolite repression control) partially relieves YE repression. Macroarray experiments show that crc transcription is strongly increased in the presence of YE, inversely correlated with TOL pathway expression. On the other hand, we have found that induced levels of expression from Pu and P(S) in the presence of YE are partially derepressed in a ptsN mutant of P. putida. PtsN but not Crc seems to directly interfere with XylR activation at target promoters. The effect of the double mutation in ptsN and crc is not the sum of the effects of each independent mutation and suggests that both regulators are elements of a common regulatory pathway. Basal expression levels from these promoters in the absence of inducer are still XylR dependent and are also repressed in the presence of yeast extract. Neither crc nor ptsN could relieve this repression.
Figures



References
-
- Abril, M. A., M. Buck, and J. L. Ramos. 1991. Activation of the Pseudomonas TOL plasmid upper pathway operon. Identification of binding sites for the positive regulator XylR and for integration host factor protein. J. Biol. Chem. 266:15832-15838. - PubMed
-
- Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. - PubMed
-
- Bertoni, G., S. Marqués, and V. de Lorenzo. 1998. Activation of the toluene-responsive regulator XylR causes a transcriptional switch between sigma54 and sigma70 promoters at the divergent PR/PS region of the TOL plasmid. Mol. Microbiol. 27:651-659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

