Rapid detection of enteroviruses in small volumes of natural waters by real-time quantitative reverse transcriptase PCR
- PMID: 16085845
- PMCID: PMC1183282
- DOI: 10.1128/AEM.71.8.4523-4530.2005
Rapid detection of enteroviruses in small volumes of natural waters by real-time quantitative reverse transcriptase PCR
Abstract
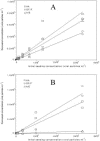
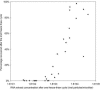
Despite viral contamination of recreational waters, only bacterial, not viral, indicators are monitored routinely, due to a lack of rapid and cost-effective assays. We used negatively charged filters to capture enteroviruses from seawater and freshwater. Viral RNA was extracted using a commercial kit, and the viruses were quantified by real-time quantitative reverse transcriptase PCR (qRT-PCR). Poliovirus (6.6 to 330,000 virus particles/ml) was added to samples from watersheds in Los Angeles, California, and analysis showed that with 50-ml samples, a cellulose acetate/nitrate (HA) filter yielded final recovery of 51% (r2= 0.99) in fresh water and 23% (r2= 0.90) in seawater. However, for additions of low levels of virus (more likely to represent field samples; <10(4) enterovirus particles/ml), the recovery was lower and more variable, with HA being best in freshwater (17%, r2= 0.97) and the type GF/F glass filter having higher average recovery in seawater (GF/F, 17%; r2= 0.93; HA 12%, r2= 0.87). The optimized method was used with 1-liter field samples from two very different freshwater "creeks" that drain into Santa Monica Bay, California: Topanga Creek (TC), a relatively pristine mountain creek, and Ballona Creek (BC), a concrete-lined urban storm drain. One TC site out of 10 and 2 BC sites out of 7 tested significantly positive for enteroviruses, with higher enterovirus concentrations in BC than in TC (ca. 10 to 25 versus 1 equivalent enterovirus particle/ml). The presented filtration-qRT-PCR approach is fast (<8 h from sampling to results), sensitive, and cost efficient and is promising for monitoring viral contamination in environmental water samples.
Figures



References
-
- Boehm, A. B., J. A. Fuhrman, R. D. Mrše, and S. B. Grant. 2003. A tiered approach for identification of a human fecal pollution source at a recreational beach: case study at Avalon Bay, Catalina Island, California, USA. Env. Sci. Technol. 37:673-680. - PubMed
-
- Davis, B. D., R. Dulbecco, H. N. Eisen, and H. S. Ginsberg. 1990. Microbiology, 4th ed. J. B. Lippincott, Philadelphia, Pa.
-
- Donaldson, K. A., D. W. Griffin, and J. H. Paul. 2002. Detection, quantitation and identification of enteroviruses from surface waters and sponge tissue from the Florida Keys using real-time RT-PCR. Water Res. 36:2505-2514. - PubMed
-
- Enriquez, C. E., M. Abbaszadegan, I. L. Pepper, K. J. Richardson, and C. P. Gerba. 1993. Poliovirus detection in water by cell culture and nucleic-acid hybridization. Water Res. 27:1113-1118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

