Regiospecificity of two multicomponent monooxygenases from Pseudomonas stutzeri OX1: molecular basis for catabolic adaptation of this microorganism to methylated aromatic compounds
- PMID: 16085870
- PMCID: PMC1183305
- DOI: 10.1128/AEM.71.8.4736-4743.2005
Regiospecificity of two multicomponent monooxygenases from Pseudomonas stutzeri OX1: molecular basis for catabolic adaptation of this microorganism to methylated aromatic compounds
Abstract
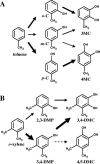
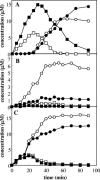
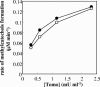
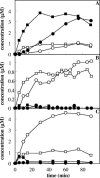
The pathways for degradation of aromatic hydrocarbons are constantly modified by a variety of genetic mechanisms. Genetic studies carried out with Pseudomonas stutzeri OX1 suggested that the tou operon coding for toluene o-xylene monooxygenase (ToMO) was recently recruited into a preexisting pathway that already possessed the ph operon coding for phenol hydroxylase (PH). This apparently resulted in a redundancy of enzymatic activities, because both enzymes are able to hydroxylate (methyl)benzenes to (methyl)catechols via the intermediate production of (methyl)phenols. We investigated the kinetics and regioselectivity of toluene and o-xylene oxidation using Escherichia coli cells expressing ToMO and PH complexes. Our data indicate that in the recombinant system the enzymes act sequentially and that their catalytic efficiency and regioselectivity optimize the degradation of toluene and o-xylene, both of which are growth substrates. The main product of toluene oxidation by ToMO is p-cresol, the best substrate for PH, which catalyzes its transformation to 4-methylcatechol. The sequential action of the two enzymes on o-xylene leads, via the intermediate 3,4-dimethylphenol, to the exclusive production of 3,4-dimethylcatechol, the only dimethylcatechol isomer that can serve as a carbon and energy source after further metabolic processing. Moreover, our data strongly support a metabolic explanation for the acquisition of the ToMO operon by P. stutzeri OX1. It is possible that using the two enzymes in a concerted fashion confers on the strain a selective advantage based on the ability of the microorganism to optimize the efficiency of the use of nonhydroxylated aromatic hydrocarbons, such as benzene, toluene, and o-xylene.
Figures
Similar articles
-
Mutation of glutamic acid 103 of toluene o-xylene monooxygenase as a means to control the catabolic efficiency of a recombinant upper pathway for degradation of methylated aromatic compounds.Appl Environ Microbiol. 2005 Aug;71(8):4744-50. doi: 10.1128/AEM.71.8.4744-4750.2005. Appl Environ Microbiol. 2005. PMID: 16085871 Free PMC article.
-
Phenol hydroxylase and toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1: interplay between two enzymes.Appl Environ Microbiol. 2004 Apr;70(4):2211-9. doi: 10.1128/AEM.70.4.2211-2219.2004. Appl Environ Microbiol. 2004. PMID: 15066815 Free PMC article.
-
Organization and regulation of meta cleavage pathway genes for toluene and o-xylene derivative degradation in Pseudomonas stutzeri OX1.Appl Environ Microbiol. 2001 Jul;67(7):3304-8. doi: 10.1128/AEM.67.7.3304-3308.2001. Appl Environ Microbiol. 2001. PMID: 11425758 Free PMC article.
-
Substrate trafficking and dioxygen activation in bacterial multicomponent monooxygenases.Acc Chem Res. 2007 Jul;40(7):466-74. doi: 10.1021/ar600040e. Epub 2007 May 23. Acc Chem Res. 2007. PMID: 17518435 Review.
-
Phylogenetic and Functional Diversity of Soluble Di-Iron Monooxygenases.Environ Microbiol. 2025 Feb;27(2):e70050. doi: 10.1111/1462-2920.70050. Environ Microbiol. 2025. PMID: 39947201 Free PMC article. Review.
Cited by
-
Degradation of toluene by ortho cleavage enzymes in Burkholderia fungorum FLU100.Microb Biotechnol. 2015 Jan;8(1):143-54. doi: 10.1111/1751-7915.12147. Epub 2014 Aug 18. Microb Biotechnol. 2015. PMID: 25130674 Free PMC article.
-
X-ray structure of a hydroxylase-regulatory protein complex from a hydrocarbon-oxidizing multicomponent monooxygenase, Pseudomonas sp. OX1 phenol hydroxylase.Biochemistry. 2006 Dec 26;45(51):15392-404. doi: 10.1021/bi0618969. Epub 2006 Dec 2. Biochemistry. 2006. PMID: 17176061 Free PMC article.
-
Mutation of glutamic acid 103 of toluene o-xylene monooxygenase as a means to control the catabolic efficiency of a recombinant upper pathway for degradation of methylated aromatic compounds.Appl Environ Microbiol. 2005 Aug;71(8):4744-50. doi: 10.1128/AEM.71.8.4744-4750.2005. Appl Environ Microbiol. 2005. PMID: 16085871 Free PMC article.
-
Toward functional carboxylate-bridged diiron protein mimics: achieving structural stability and conformational flexibility using a macrocylic ligand framework.J Am Chem Soc. 2011 Jul 13;133(27):10568-81. doi: 10.1021/ja2021312. Epub 2011 Jun 17. J Am Chem Soc. 2011. PMID: 21682286 Free PMC article.
-
Discovery of an Inducible Toluene Monooxygenase That Cooxidizes 1,4-Dioxane and 1,1-Dichloroethylene in Propanotrophic Azoarcus sp. Strain DD4.Appl Environ Microbiol. 2020 Aug 18;86(17):e01163-20. doi: 10.1128/AEM.01163-20. Print 2020 Aug 18. Appl Environ Microbiol. 2020. PMID: 32591384 Free PMC article.
References
-
- Assinder, S. J., and P. A. Williams. 1990. The TOL plasmids: determinants of the catabolism of toluene and xylenes. Adv. Microb. Physiol. 31:1-69. - PubMed
-
- Barbieri, P., F. L. Arenghi, G. Bertoni, F. Bolognese, and E. Galli. 2001. Evolution of catabolic pathways and metabolic versatility in Pseudomonas stutzeri OX1. Antonie Leeuwenhoek 79:135-140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

