Mutation of glutamic acid 103 of toluene o-xylene monooxygenase as a means to control the catabolic efficiency of a recombinant upper pathway for degradation of methylated aromatic compounds
- PMID: 16085871
- PMCID: PMC1183268
- DOI: 10.1128/AEM.71.8.4744-4750.2005
Mutation of glutamic acid 103 of toluene o-xylene monooxygenase as a means to control the catabolic efficiency of a recombinant upper pathway for degradation of methylated aromatic compounds
Abstract
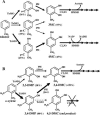
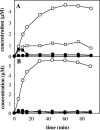
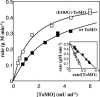
Toluene o-xylene monooxygenase (ToMO) and phenol hydroxylase (PH) of Pseudomonas stutzeri OX1 act sequentially in a recombinant upper pathway for the degradation of aromatic hydrocarbons. The catalytic efficiency and regioselectivity of these enzymes optimize the degradation of growth substrates like toluene and o-xylene. For example, the sequential monooxygenation of o-xylene by ToMO and PH leads to almost exclusive production of 3,4-dimethylcatechol (3,4-DMC), the only isomer that can be further metabolized by the P. stutzeri meta pathway. We investigated the possibility of producing ToMO mutants with modified regioselectivity compared with the regioselectivity of the wild-type protein in order to alter the ability of the recombinant upper pathway to produce methylcatechol isomers from toluene and to produce 3,4-DMC from o-xylene. The combination of mutant (E103G)-ToMO and PH increased the production of 4-methylcatechol from toluene and increased the formation of 3,4-DMC from o-xylene. These data strongly support the idea that the products and efficiency of the metabolic pathway can be controlled not only through mutations that increase the catalytic efficiency of the enzymes involved but also through tuning the substrate specificity and regioselectivity of the enzymes. These findings are crucial for the development of future metabolic engineering strategies.
Figures
References
-
- Fetzner, S., and J. R. van der Meer. 2000. Enzymes involved in the aerobic bacterial degradation of N-heteroaromatic compounds: molybdenum hydroxylases and ring-opening 2,4-dioxygenases. Naturwissenschaften 87:59-69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

