Insights into the biosynthesis of the benzoquinone ansamycins geldanamycin and herbimycin, obtained by gene sequencing and disruption
- PMID: 16085885
- PMCID: PMC1183368
- DOI: 10.1128/AEM.71.8.4862-4871.2005
Insights into the biosynthesis of the benzoquinone ansamycins geldanamycin and herbimycin, obtained by gene sequencing and disruption
Abstract
Geldanamycin and the closely related herbimycins A, B, and C were the first benzoquinone ansamycins to be extensively studied for their antitumor properties as small-molecule inhibitors of the Hsp90 protein chaperone complex. These compounds are produced by two different Streptomyces hygroscopicus strains and have the same modular polyketide synthase (PKS)-derived carbon skeleton but different substitution patterns at C-11, C-15, and C-17. To set the stage for structural modification by genetic engineering, we previously identified the gene cluster responsible for geldanamycin biosynthesis. We have now cloned and sequenced a 115-kb segment of the herbimycin biosynthetic gene cluster from S. hygroscopicus AM 3672, including the genes for the PKS and most of the post-PKS tailoring enzymes. The similarities and differences between the gene clusters and biosynthetic pathways for these closely related ansamycins are interpreted with support from the results of gene inactivation experiments. In addition, the organization and functions of genes involved in the biosynthesis of the 3-amino-5-hydroxybenzoic acid (AHBA) starter unit and the post-PKS modifications of progeldanamycin were assessed by inactivating the subclusters of AHBA biosynthetic genes and two oxygenase genes (gdmM and gdmL) that were proposed to be involved in formation of the geldanamycin benzoquinoid system. A resulting novel geldanamycin analog, KOS-1806, was isolated and characterized.
Figures


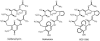
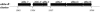
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Arakawa, K., R. Muller, T. Mahmud, T. W. Yu, and H. G. Floss. 2002. Characterization of the early stage aminoshikimate pathway in the formation of 3-amino-5-hydroxybenzoic acid: the RifN protein specifically converts kanosamine into kanosamine 6-phosphate. J. Am. Chem. Soc. 124:10644-10645. - PubMed
-
- August, P. R., L. Tang, Y. J. Yoon, S. Ning, R. Muller, T. W. Yu, M. Taylor, D. Hoffmann, C. G. Kim, X. Zhang, C. R. Hutchinson, and H. G. Floss. 1998. Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 5:69-79. - PubMed
-
- Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. - PubMed
-
- Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the omega interposon for use in Escherichia coli and Streptomyces. Gene 190:315-317. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

