Two-dimensional measurement of proton T1rho relaxation in unlabeled proteins: mobility changes in alpha-bungarotoxin upon binding of an acetylcholine receptor peptide
- PMID: 16086595
- PMCID: PMC2597414
- DOI: 10.1021/bi050645h
Two-dimensional measurement of proton T1rho relaxation in unlabeled proteins: mobility changes in alpha-bungarotoxin upon binding of an acetylcholine receptor peptide
Abstract
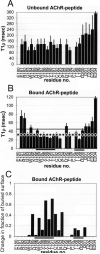
A method for the measurement of proton T(1)(rho) relaxation times in unlabeled proteins is described using a variable spin-lock pulse after the initial nonselective 90 degrees excitation in a HOHAHA pulse sequence. The experiment is applied to alpha-bungarotoxin (alpha-BTX) and its complex with a 25-residue peptide derived from the acetylcholine receptor (AChR) alpha-subunit. A good correlation between high T(1)(rho) values and increased local motion is revealed. In the free form, toxin residues associated with receptor binding according to the NMR structure of the alpha-BTX complex with an AChR peptide and the model for alpha-BTX with the AChR [Samson, A. O., et al. (2002) Neuron 35, 319-332] display high mobility. When the AChR peptide binds, a decrease in the relaxation times and the level of motion of residues involved in binding of the receptor alpha-subunit is exhibited, while residues implicated in binding gamma- and delta-subunits retain their mobility. In addition, the quantitative T(1)(rho) measurements enable us to corroborate the mapping of boundaries of the AChR determinant strongly interacting with the toxin [Samson, A. O., et al. (2001) Biochemistry 40, 5464-5473] and can similarly be applied to other protein complexes in which peptides represent one of the two interacting proteins. The presented method is advantageous because of its simplicity, generality, and time efficiency and paves the way for future investigation of proton relaxation rates in small unlabeled proteins.
Figures





References
-
- Torchia D, Lyerla J, Quattrone AJ. Molecular dynamics and structure of the random coil and helical states of the collagen peptide, α1-CB2, as determined by 13C magnetic resonance. Biochemistry. 1975;14:887–900. - PubMed
-
- Jardetzky O, Roberts GCK. NMR in molecular biology. Academic Press, Inc.; New York: 1981.
-
- Ishima R, Wingfield PT, Stahl SJ, Kaufman JD, Torchia DA. Using amide 1H and 15N transverse relaxation to detect millisecond time-scale motions in perdeuterated proteins: application to HIV-1 protease. J. Am. Chem. Soc. 1998;120:10534–42.
-
- Boulat B, Bodenhausen G. Measurement of proton relaxation rates in proteins. J. Biomol. NMR. 1993;3:335–348.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

