Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A
- PMID: 16087679
- PMCID: PMC1762002
- DOI: 10.1074/jbc.M505229200
Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A
Abstract
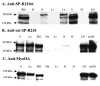
Mass spectrometric characterization of the surfactant protein A (SP-A) receptor 210 (SP-R210) led to the identification of myosin (Myo) XVIIIA and nonmuscle myosin IIA. Antibodies generated against the unique C-terminal tail of MyoXVIIIA revealed that MyoXVIIIA, MyoIIA, and SP-R210 have overlapping tissue distribution, all being highly expressed in myeloid cells, bone marrow, spleen, lymph nodes, and lung. Western blot analysis of COS-1 cells stably transfected with either MyoXVIIIA or MyoIIA indicated that SP-R210 antibodies recognize MyoXVIIIA. Furthermore, MyoXVIIIA but not MyoIIA localized to the surface of COS-1 cells, and most importantly, expression of MyoXVIIIA in COS-1 cells conferred SP-A binding. Western analysis of recombinant MyoXVIIIA domains expressed in bacteria mapped the epitopes of previously derived SP-R210 antibodies to the neck region of MyoXVIIIA. Antibodies raised against the neck domain of MyoXVIIIA blocked the binding of SP-A to macrophages. Together, these findings indicate that MyoXVIIIA constitutes a novel receptor for SP-A.
Figures







References
-
- van de Wetering JK, van Golde LM, Batenburg JJ. Eur. J. Biochem. 2004;271:1229–1249. - PubMed
-
- Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. J. Immunol. 2003;171:417–425. - PubMed
-
- Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, SiTahar M. J. Immunol. 2002;168:5989–5992. - PubMed
-
- Hohlfeld JM, Erpenbeck VJ, Krug N. Pathobiology. 2002;70:287–292. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

