Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways
- PMID: 16087710
- PMCID: PMC2171499
- DOI: 10.1083/jcb.200506051
Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways
Abstract
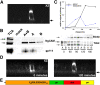
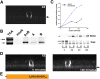
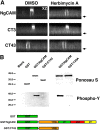
NgCAM is a cell adhesion molecule that is largely axonal in neurons and apical in epithelia. In Madin-Darby canine kidney cells, NgCAM is targeted to the apical surface by transcytosis, being first inserted into the basolateral domain from which it is internalized and transported to the apical domain. Initial basolateral transport is mediated by a sequence motif (Y(33)RSL) decoded by the AP-1B clathrin adaptor complex. This motif is a substrate in vitro for tyrosine phosphorylation by p60src, a modification that disrupts NgCAM's ability to interact with clathrin adaptors. Based on the behavior of various NgCAM mutants, it appears that after arrival at the basolateral surface, the AP-1B interaction site is silenced by phosphorylation of Tyr(33). This slows endocytosis and inhibits basolateral recycling from endosomes, resulting in NgCAM transcytosis due to a cryptic apical targeting signal in its extracellular domain. Thus, transcytosis of NgCAM and perhaps other membrane proteins may reflect the spatial regulation of recognition by adaptors such as AP-1B.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

