Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae
- PMID: 16087741
- PMCID: PMC1214537
- DOI: 10.1128/EC.4.8.1364-1374.2005
Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae
Abstract
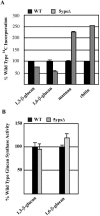
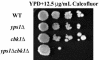
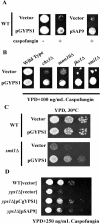
The yeast cell wall is a crucial extracellular organelle that protects the cell from lysis during environmental stress and morphogenesis. Here, we demonstrate that the yapsin family of five glycosylphosphatidylinositol-linked aspartyl proteases is required for cell wall integrity in Saccharomyces cerevisiae. Yapsin null mutants show hypersensitivity to cell wall perturbation, and both the yps1Delta2Delta mutant and the quintuple yapsin mutant (5ypsDelta) undergo osmoremedial cell lysis at 37 degrees C. The cell walls of both 5ypsDelta and yps1Delta2Delta mutants have decreased amounts of 1,3- and 1,6-beta-glucan. Although there is decreased incorporation of both 1,3- and 1,6-beta-glucan in the 5ypsDelta mutant in vivo, in vitro specific activity of both 1,3- and 1,6-beta-glucan synthesis is similar to wild type, indicating that the yapsins affect processes downstream of glucan synthesis and that the yapsins may be involved in the incorporation or retention of cell wall glucan. Presumably as a response to the significant alterations in cell wall composition, the cell wall integrity mitogen-activated kinase signaling cascade (PKC1-MPK pathway) is basally active in 5ypsDelta. YPS1 expression is induced during cell wall stress and remodeling in a PKC1-MPK1-dependent manner, indicating that Yps1p is a direct, and important, output of the cell wall integrity response. The Candida albicans (SAP9) and Candida glabrata (CgYPS1) homologues of YPS1 complement the phenotypes of the yps1Delta mutant. Taken together, these data indicate that the yapsins play an important role in glucan homeostasis in S. cerevisiae and that yapsin homologues may play a similar role in the pathogenic yeasts C. albicans and C. glabrata.
Figures



References
-
- Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. S. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. - PubMed
-
- Ash, J., M. Dominguez, J. J. M. Bergeron, D. Y. Thomas, and Y. Boubonnais. 1995. The yeast proprotein convertase encoded by YAP3 is a glycophosphatidylinositol-anchored protein that localizes to the plasma membrane. J. Biol. Chem. 270:20847-20854. - PubMed
-
- Basco, R. D., G. Gimenez-Gallego, and G. Larriba. 1990. Processing of yeast exoglucanase (beta-glucosidase) in a KEX2-dependent manner. FEBS Lett. 268:99-102. - PubMed
-
- Burke, D. J., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Woodbury, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

