Ubiquinone synthesis in mitochondrial and microsomal subcellular fractions of Pneumocystis spp.: differential sensitivities to atovaquone
- PMID: 16087753
- PMCID: PMC1214522
- DOI: 10.1128/EC.4.8.1483-1492.2005
Ubiquinone synthesis in mitochondrial and microsomal subcellular fractions of Pneumocystis spp.: differential sensitivities to atovaquone
Abstract
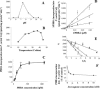
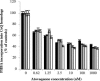
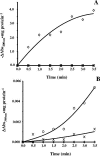
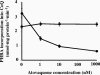
The lung pathogen Pneumocystis spp. is the causative agent of a type of pneumonia that can be fatal in people with defective immune systems, such as AIDS patients. Atovaquone, an analog of ubiquinone (coenzyme Q [CoQ]), inhibits mitochondrial electron transport and is effective in clearing mild to moderate cases of the infection. Purified rat-derived intact Pneumocystis carinii cells synthesize de novo four CoQ homologs, CoQ7, CoQ8, CoQ9, and CoQ10, as demonstrated by the incorporation of radiolabeled precursors of both the benzoquinone ring and the polyprenyl chain. A central step in CoQ biosynthesis is the condensation of p-hydroxybenzoic acid (PHBA) with a long-chain polyprenyl diphosphate molecule. In the present study, CoQ biosynthesis was evaluated by the incorporation of PHBA into completed CoQ molecules using P. carinii cell-free preparations. CoQ synthesis in whole-cell homogenates was not affected by the respiratory inhibitors antimycin A and dicyclohexylcarbodiimide but was diminished by atovaquone. Thus, atovaquone has inhibitory activity on both electron transport and CoQ synthesis in this pathogen. Furthermore, both the mitochondrial and microsomal fractions were shown to synthesize de novo all four P. carinii CoQ homologs. Interestingly, atovaquone inhibited microsomal CoQ synthesis, whereas it had no effect on mitochondrial CoQ synthesis. This is the first pathogenic eukaryotic microorganism in which biosynthesis of CoQ molecules from the initial PHBA:polyprenyl transferase reaction has been unambiguously shown to occur in two distinct compartments of the same cell.
Figures



Similar articles
-
Ubiquinone synthesis and its regulation in Pneumocystis carinii.J Eukaryot Microbiol. 2006 Nov-Dec;53(6):435-44. doi: 10.1111/j.1550-7408.2006.00127.x. J Eukaryot Microbiol. 2006. PMID: 17123407
-
Pneumocystis carinii f. sp. carinii synthesizes de novo four homologs of ubiquinone.J Eukaryot Microbiol. 2001 Mar-Apr;48(2):182-7. doi: 10.1111/j.1550-7408.2001.tb00301.x. J Eukaryot Microbiol. 2001. PMID: 12095106
-
Ubiquinone synthesis by Pneumocystis carinii: incorporation of radiolabeled polyprenyl chain and benzoquinone ring precursors.J Eukaryot Microbiol. 1997 Nov-Dec;44(6):60S. doi: 10.1111/j.1550-7408.1997.tb05780.x. J Eukaryot Microbiol. 1997. PMID: 9508445
-
Are cytochrome b gene mutations the only cause of atovaquone resistance in Pneumocystis?Drug Resist Updat. 2001 Oct;4(5):322-9. doi: 10.1054/drup.2001.0221. Drug Resist Updat. 2001. PMID: 11991686 Review.
-
Coenzyme Q biosynthesis in health and disease.Biochim Biophys Acta. 2016 Aug;1857(8):1079-1085. doi: 10.1016/j.bbabio.2016.03.036. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060254 Review.
Cited by
-
Confirmation of emergence of mutations associated with atovaquone-proguanil resistance in unexposed Plasmodium falciparum isolates from Africa.Malar J. 2006 Oct 4;5:82. doi: 10.1186/1475-2875-5-82. Malar J. 2006. PMID: 17020611 Free PMC article.
References
-
- Barr, R., B. A. Branstetter, A. Rajnicek, F. L. Crane, and H. Low. 1991. Chloroquine-sensitive transplasmalemma electron transport in Tetrahymena pyriformis: a hypothesis for control of parasite protozoa through transmembrane redox. Biochim. Biophys. Acta 1058:261-268. - PubMed
-
- Bonner, W. D. 1955. Succinic dehydrogenase. Methods Enzymol. 1:722-725.
-
- Cirioni, O., A. Giacometti, and G. Scalise. 1997. In vitro activity of atovaquone, sulphamethoxazole and dapsone alone and combined with inhibitors of dihydrofolate reductase and macrolides against Pneumocystis carinii. J. Antimicrob. Chemother. 39:45-51. - PubMed
-
- Córdoba-Pedregosa, M. del, C., J. M. Villalba, and F. J. Alcaín. 2005. Determination of coenzyme Q biosynthesis in cultured cells without the necessity for lipid extraction. Anal. Biochem. 336:60-63. - PubMed
-
- Crane, F. L., I. L. Sun, and E. E. Sun. 1993. The essential functions of coenzyme Q. Clin. Investig. 71(8 Suppl.):zfpg>S55-S59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

