Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching
- PMID: 16087884
- PMCID: PMC1187956
- DOI: 10.1073/pnas.0500781102
Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching
Abstract
The developmental basis of morphological complexity remains a central question in developmental and evolutionary biology. Feathers provide a unique system to analyze the development of complex morphological novelties. Here, we describe the interactions between Sonic hedgehog (Shh) and bone morphogenetic protein 2 (Bmp2) signaling during feather barb ridge morphogenesis. We demonstrate that activator-inhibitor models of Shh and Bmp2 signaling in the tubular feather epithelium are sufficient to explain the initial formation of a meristic pattern of barb ridges and the observed variation in barb morphogenesis in chick natal down feathers. Empirical tests support the assumptions of the model that, within the feather ectoderm, Shh (activator) up-regulates its own transcription and that of Bmp2 (inhibitor), whereas Bmp2 signaling down-regulates Shh expression. More complex models incorporating a second activator and dorsal/ventral polarized modification of activator signaling can produce all of the barb morphogenesis patterns observed during the growth of more complex branched pennaceous feathers: new barb ridge formation, helical growth, and barb ridge fusion. An integrated model of feather morphogenesis and evolution suggests that plumulaceous feather structure evolved by the establishment of activator-inhibitor interactions between Shh and Bmp2 signaling in the basal epithelium of the feather germ. Subsequently, pennaceous feather structure evolved through the integration of barb ridge morphogenesis with a second, local inhibitor and a dorsal/ventral signal gradient within the feather. The model is congruent with paleontological evidence that plumulaceous feathers are primitive to pennaceous feathers.
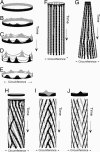
Figures


References
-
- Prum, R. O. (1999) J. Exp. Zool. Mol. Dev. Evol. 285, 291–306. - PubMed
-
- Prum, R. O. & Dyck, J. (2003) J. Exp. Zool. B Mol. Dev. Evol. 298, 73–90. - PubMed
-
- Lucas, A. M. & Stettenheim, P. R. (1972) Avian Anatomy-Integument (U.S. Department of Agriculture Handbook, Washington, D.C.).
-
- Stettenheim, P. (1976) Proc. 16th Intl. Ornitholog. Congr. 16, 385–401.
-
- Prum, R. O. & Williamson, S. (2001) J. Exp. Zool. Mol. Dev. Evol. 291, 30–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

