Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis
- PMID: 16087887
- PMCID: PMC1188016
- DOI: 10.1073/pnas.0505622102
Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis
Abstract
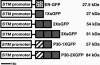
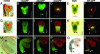
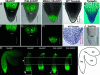
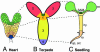
The axial body pattern of Arabidopsis is determined during embryogenesis by auxin signaling and differential gene expression. Here we demonstrate that another pathway, cell-to-cell communication through plasmodesmata (PD), is regulated during apical-basal pattern formation. The SHOOT MERISTEMLESS (STM) promoter was used to drive expression in the shoot apical meristem (SAM) and a subset of cells at the base of the hypocotyl of 1x,2x, and 3x soluble green fluorescent proteins (sGFPs), and the P30 movement protein of Tobacco mosaic virus (TMV) translationally fused to 1x and 2x sGFP. In the early heart stage, 2x sGFP (54 kDa) moves throughout the whole embryo, whereas 3x sGFP (81 kDa) shows more restricted movement. As the embryo develops, PD apertures are down regulated to form local subdomains allowing transport of different sized tracers. For example, movement of 2x sGFP to the cotyledon, and 3x sGFP to root tips, becomes restricted. Subdomains of cell-to-cell transport align with the apical-basal embryo body axis and correspond to the shoot apex, cotyledons, hypocotyl, and root. Studies with P30-GFP fusions reinforce the distinction between embryonic symplastic subdomains. Although P30 targets embryo cell walls as puncta (diagnostic for functional localization of P30 to PD in adult plants), P30 cannot dilate embryonic PD to overcome the barriers for transport between symplastic subdomains, suggesting that specific boundaries separate symplastic subdomains of the embryo. Thus, cell-to-cell communication via plasmodesmata conveys positional information critical to establish the axial body pattern during embryogenesis in Arabidopsis.
Figures


References
-
- Scheres, B., Wolkenfelt, H., Willemsen, V., Terlouw, M., Lawson, E., Dean, C. & Weisbeek, P. (1994) Development (Cambridge, U.K.) 120, 2475-2487.
-
- Poethig, R., Coe, E. & Johri, M. (1986) Dev. Biol. 117, 392-404.
-
- Saulsberry, A., Martin, P. R., O'Brien, T., Sieburth, L. E. & Pickett, F. B. (2002) Development (Cambridge, U.K.) 129, 3403-3410. - PubMed
-
- Berleth, T. & Chatfield, S. (2002) in The Arabidopsis Book, eds. Somerville, C. & Meyerowitz, E. (Am. Soc. Plant. Biol., Rockville, MD), pp. 1-22.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

