Effects of recombinant hematopoietins on blood-loss anemia in mice
- PMID: 16089085
- PMCID: PMC1888772
Effects of recombinant hematopoietins on blood-loss anemia in mice
Abstract
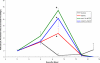
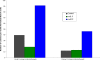
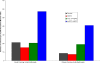
Use of recombinant human erythropoietin (rhEPO) for treatment of pre-operative anemia in anticipation of orthopaedic surgical blood loss has become a routine practice. Use of rhEPO to help manage unanticipated blood loss from elective surgery or major orthopaedic trauma is limited by the rate and volume of erythropoiesis that is achievable with exogenously administered rhEPO. The rate and volume of erythropoiesis may be limited by the available population of cells responsive to EPO. Cytokines known to affect these early hematopoietic progenitors may potentiate the effects of rhEPO. In this study, mice were rendered anemic by loss of approximately one-third of their total blood volume. A control group received only iron supplementation. Mice in three experimental groups received three injections of rhEPO. Two of these groups also received either recombinant murine stem cell factor (rmSCF) or recombinant murine interleukin-3 (rmIL-3). Both were before and in conjunction with rhEPO. Animals were sacrificed for peripheral blood testing at baseline, after initiation of rmSCF and rmIL-3 prior to rhEPO administration, and at three time points after dosing of rhEPO. Additionally, the bone marrow was harvested and cultured to determine the concentration of erythroid progenitors after treatment with rmIL-3 or rmSCF, and after further treatment with rhEPO. Hematocrits were significantly higher in the first measurement point after administration of rhEPO in the groups receiving additional cytokines. The control and rhEPO-only groups were not different at this early time point. The maximal rate of erythropoiesis was also elevated in the groups receiving additional cytokines. The bone marrow of mice receiving SCF had a dramatically increased number of erythroid progenitors compared to all other groups. The population of EPO-responsive cells, dependent on cytokines not controlled by hypoxia, is a major rate-limiting and volume-limiting factor in the response to rhEPO during recovery from blood-loss anemia. Administration of earlier-acting cytokines has the potential to increase the rate and volume of exogenously stimulated erythropoiesis.
Figures


References
-
- Dodd RY. Current safety of the blood supply in the United States. Int J Hematol. 2004;80(4):301–305. - PubMed
-
- Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg. (Am) 1999;81(1):2–10. - PubMed
-
- Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol. 1997;34(3 Suppl 2):34–40. - PubMed
-
- Borghi B, Casati A. Incidence and risk factors for allogenic blood transfusion during major joint replacement using an integrated autotransfusion regimen. Eur J Anaesthesiol. 2000;17(7):411–417. The Rizzoli Study Group on Orthopaedic Anaesthesia. - PubMed
-
- Innerhofer P, Walleczek C, Luz G, Hobisch-Hagen P, Benzer A, Stockl B, Hessenberger G, Nussbaumer W, Schobersberger W. Transfusion of buffy coat-depleted blood components and risk of postoperative infection in orthopedic patients. Transfusion. 1999;39(6):625–632. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
