Evidence for ineffective erythropoiesis in severe sickle cell disease
- PMID: 16091448
- PMCID: PMC1895054
- DOI: 10.1182/blood-2005-04-1376
Evidence for ineffective erythropoiesis in severe sickle cell disease
Abstract
Peripheral destruction of sickled erythrocytes is a cardinal feature of sickle cell disease (SCD). Less well established is the potential contribution of ineffective erythropoiesis to the pathophysiology of this hemoglobinopathy. Since patients with SCD frequently develop mixed hematopoietic chimerism after allogeneic nonmyeloablative stem cell transplantation, we used this opportunity to directly compare the differentiation and survival of SCD and donor-derived erythropoiesis in vivo. Donor and recipient erythropoiesis was compared in 4 patients with SCD and 4 without SCD who developed stable mixed hematopoietic chimerism following transplant. Molecular analysis of chimerism in peripheral blood and bone marrow demonstrated higher expression of donor-derived beta-globin RNA relative to the level of donor-derived genomic DNA in patients with SCD. Analysis of chimerism in immature (glycophorin A-positive [GYPA(+)], CD71(hi)) and mature (GYPA(+), CD71(neg)) erythroblasts confirmed the intramedullary loss of SS erythroblasts with progressive maturation. In patients with SCD, relative enrichment of donor erythroid precursors began to appear at the onset of hemoglobinization. Ineffective erythropoiesis of homozygous hemoglobin S (SS) progenitors thus provides a maturation advantage for homozygous hemoglobin A (AA) or heterozygous hemoglobin S/hemoglobin A (SA) donor erythroid precursor cells that results in greater donor contribution to overall erythropoiesis following stem-cell transplantation and improvement of clinical disease.
Figures
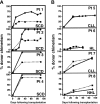
 , Beta-globin RNA chimerism.
, Beta-globin RNA chimerism.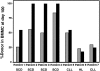
 ) and β-globin RNA (▪) donor chimerism derived from bone marrow mononuclear cells (BMMCs) were compared between patients with SCD (patients 1-4) or other diseases (patients 5-8) at day 100 following allogeneic stem cell transplantation.
) and β-globin RNA (▪) donor chimerism derived from bone marrow mononuclear cells (BMMCs) were compared between patients with SCD (patients 1-4) or other diseases (patients 5-8) at day 100 following allogeneic stem cell transplantation.
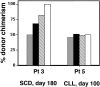
 ), immature (▪), and mature (
), immature (▪), and mature ( ) marrow erythroblasts isolated from marrow and peripheral circulating nucleated RBCs (□) between SCD patient 3 and non-SCD patient 5.
) marrow erythroblasts isolated from marrow and peripheral circulating nucleated RBCs (□) between SCD patient 3 and non-SCD patient 5.Similar articles
-
Mixed chimerism following in utero hematopoietic stem cell transplantation in murine models of hemoglobinopathy.Exp Hematol. 2003 Feb;31(2):176-84. doi: 10.1016/s0301-472x(02)01024-x. Exp Hematol. 2003. PMID: 12591283
-
Molecular assessment of erythroid lineage chimerism following nonmyeloablative allogeneic stem cell transplantation.Exp Hematol. 2003 Oct;31(10):924-33. doi: 10.1016/s0301-472x(03)00227-3. Exp Hematol. 2003. PMID: 14550808
-
Mixed donor chimerism following stem cell transplantation for sickle cell disease.Curr Opin Hematol. 2023 Nov 1;30(6):187-193. doi: 10.1097/MOH.0000000000000786. Epub 2023 Sep 1. Curr Opin Hematol. 2023. PMID: 37694765 Review.
-
Human CD34(+) and CD34(+)CD38(-) hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells.Exp Hematol. 2004 May;32(5):483-93. doi: 10.1016/j.exphem.2004.02.003. Exp Hematol. 2004. PMID: 15145217
-
Ineffective erythropoiesis in sickle cell disease: new insights and future implications.Curr Opin Hematol. 2021 May 1;28(3):171-176. doi: 10.1097/MOH.0000000000000642. Curr Opin Hematol. 2021. PMID: 33631786 Review.
Cited by
-
Preclinical Evaluation of a Novel Lentiviral Vector Driving Lineage-Specific BCL11A Knockdown for Sickle Cell Gene Therapy.Mol Ther Methods Clin Dev. 2020 Mar 17;17:589-600. doi: 10.1016/j.omtm.2020.03.015. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32300607 Free PMC article.
-
LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis.Dev Cell. 2009 Oct;17(4):527-40. doi: 10.1016/j.devcel.2009.09.005. Dev Cell. 2009. PMID: 19853566 Free PMC article.
-
Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease.Blood. 2016 Feb 18;127(7):839-48. doi: 10.1182/blood-2015-09-618587. Epub 2016 Jan 12. Blood. 2016. PMID: 26758916 Free PMC article. Review.
-
Peripheral red blood cell split chimerism as a consequence of intramedullary selective apoptosis of recipient red blood cells in a case of sickle cell disease.Mediterr J Hematol Infect Dis. 2014 Nov 1;6(1):e2014066. doi: 10.4084/MJHID.2014.066. eCollection 2014. Mediterr J Hematol Infect Dis. 2014. PMID: 25408852 Free PMC article.
-
Genome editing of HBG1 and HBG2 to induce fetal hemoglobin.Blood Adv. 2019 Nov 12;3(21):3379-3392. doi: 10.1182/bloodadvances.2019000820. Blood Adv. 2019. PMID: 31698466 Free PMC article.
References
-
- Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16: 2817-2824. - PubMed
-
- Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91: 756-763. - PubMed
-
- McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97: 3390-3400. - PubMed
-
- Alyea EP, Kim HT, Ho V, et al. Comparative out-come of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105: 1810-1814. - PubMed
-
- Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7: 665-673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

