Fractal intermediates in the self-assembly of silicatein filaments
- PMID: 16091468
- PMCID: PMC1187983
- DOI: 10.1073/pnas.0503968102
Fractal intermediates in the self-assembly of silicatein filaments
Abstract
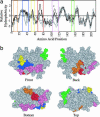
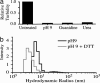
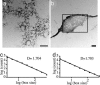
Silicateins are proteins with catalytic, structure-directing activity that are responsible for silica biosynthesis in certain sponges; they are the constituents of macroscopic protein filaments that are found occluded within the silica needles made by Tethya aurantia. Self-assembly of the silicatein monomers and oligomers is shown to form fibrous structures by a mechanism that is fundamentally different from any previously described filament-assembly process. This assembly proceeds through the formation of diffusion-limited, fractally patterned aggregates on the path to filament formation. The driving force for this self-assembly is suggested to be entropic, mediated by the interaction of hydrophobic patches on the surfaces of the silicatein subunits that are not found on highly homologous congeners that do not form filaments. Our results are consistent with a model in which silicatein monomers associate into oligomers that are stabilized by intermolecular disulfide bonds. These oligomeric units assemble into a fractal network that subsequently condenses and organizes into a filamentous structure. These results represent a potentially general mechanism for protein fiber self-assembly.
Figures



Similar articles
-
Silintaphin-1--interaction with silicatein during structure-guiding bio-silica formation.FEBS J. 2011 Apr;278(7):1145-55. doi: 10.1111/j.1742-4658.2011.08040.x. Epub 2011 Mar 4. FEBS J. 2011. PMID: 21284806
-
Silicatein: A Unique Silica-Synthesizing Catalytic Triad Hydrolase From Marine Sponge Skeletons and Its Multiple Applications.Methods Enzymol. 2018;605:429-455. doi: 10.1016/bs.mie.2018.02.025. Epub 2018 Apr 11. Methods Enzymol. 2018. PMID: 29909834
-
Fractal-related assembly of the axial filament in the demosponge Suberites domuncula: relevance to biomineralization and the formation of biogenic silica.Biomaterials. 2007 Oct;28(30):4501-11. doi: 10.1016/j.biomaterials.2007.06.030. Epub 2007 Jul 12. Biomaterials. 2007. PMID: 17628661
-
Silicateins, silicatein interactors and cellular interplay in sponge skeletogenesis: formation of glass fiber-like spicules.FEBS J. 2012 May;279(10):1721-36. doi: 10.1111/j.1742-4658.2012.08533.x. Epub 2012 Apr 17. FEBS J. 2012. PMID: 22340505 Review.
-
[Progress in silicatein from sponges].Sheng Wu Gong Cheng Xue Bao. 2009 Dec;25(12):1882-6. Sheng Wu Gong Cheng Xue Bao. 2009. PMID: 20352963 Review. Chinese.
Cited by
-
Optical properties of in-vitro biomineralised silica.Sci Rep. 2012;2:607. doi: 10.1038/srep00607. Epub 2012 Aug 29. Sci Rep. 2012. PMID: 22934130 Free PMC article.
-
Fabrication of silica on chitin in ambient conditions using silicatein fused with a chitin-binding domain.Bioprocess Biosyst Eng. 2021 Sep;44(9):1883-1890. doi: 10.1007/s00449-021-02568-w. Epub 2021 May 11. Bioprocess Biosyst Eng. 2021. PMID: 33974134
-
Improved Production and Biophysical Analysis of Recombinant Silicatein-α.Biomolecules. 2020 Aug 20;10(9):1209. doi: 10.3390/biom10091209. Biomolecules. 2020. PMID: 32825281 Free PMC article.
-
Acquisition of structure-guiding and structure-forming properties during maturation from the pro-silicatein to the silicatein form.J Biol Chem. 2012 Jun 22;287(26):22196-205. doi: 10.1074/jbc.M112.351486. Epub 2012 Apr 27. J Biol Chem. 2012. PMID: 22544742 Free PMC article.
-
Precise Protein Photolithography (P3): High Performance Biopatterning Using Silk Fibroin Light Chain as the Resist.Adv Sci (Weinh). 2017 Jul 6;4(9):1700191. doi: 10.1002/advs.201700191. eCollection 2017 Sep. Adv Sci (Weinh). 2017. PMID: 28932678 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

