Abciximab in patients with ruptured intracranial aneurysms
- PMID: 16091524
- PMCID: PMC7975173
Abciximab in patients with ruptured intracranial aneurysms
Abstract
Background and purpose: Experience with intravenous abciximab to manage thromboembolism during treatment of ruptured intracranial aneurysms is limited. We present our experience in 13 patients.
Methods: We retrospectively reviewed all patients with thromboembolic complications during endovascular management of ruptured intracranial aneurysms. Thromboembolic complications were treated with intravenous abciximab. We recorded patient and aneurysm demographics, aneurysm occlusion, drug therapy, complications, and outcomes.
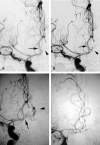
Results: World Federation of Neurological Surgeons Grades were 1 or 2 in 11 patients (85%). Median time from diagnostic angiography to treatment was 1 day. Ten (77%) aneurysms involved the anterior or posterior communicating artery, and one each occurred in the posterior inferior cerebellar artery, middle cerebral artery, and basilar regions. Eleven aneurysms were <10 mm. Five were incompletely occluded (0%-90% treated) at the time of the complication. Thromboembolic complications were at the coil-ball/parent-artery interface in nine patients (69%). Two were associated with coil-loop prolapse; one was prophylactically treated without evidence of thromboembolism. Five patients (38%) had distal complications; one also had a proximal thrombus. All patients received an intravenous bolus of abciximab (5-10 mg in 92%) without infusion. Postprocedural recanalization was complete in eight (62%) and partial in four (31%). Eleven patients (85%) had a Glasgow Outcome Scale score of 1 at 3 months. One had a poor outcome (GOS4). One died following additional coiling after abciximab administration, though this intervention was uneventful in three others.
Conclusion: Abciximab completely or partially treated thromboembolic complications arising during coiling of ruptured aneurysms. Further coiling should be performed with extreme caution and needs to be decided on a patient-by-patient basis.
Figures

Comment in
-
Platelet receptors.AJNR Am J Neuroradiol. 2006 Jan;27(1):8-9; author reply 9. AJNR Am J Neuroradiol. 2006. PMID: 16418346 Free PMC article. No abstract available.
References
-
- Tcheng JE, Ellis SG, George BS, et al. Pharmacodynamics of chimeri glycoprotein IIb/IIIa integrin antiplatelet antibody fab 7E3 in high risk coronary angioplasty. Circulation 1994;90:1757–1764 - PubMed
-
- The Abciximab in Acute Ischaemic Stroke Trial. Abciximab in acute ischaemic stroke: a randomised, double blind placebo controlled dose escalation study. Stroke 2000;31:601–609 - PubMed
-
- EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med 1994;330:956–961 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
