Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent
- PMID: 16091525
- PMCID: PMC7975144
Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent
Abstract
Background and purpose: A new neurovascular microstent, the Cordis Enterprise stent, composed of nitinol, with a closed cell design, was specifically developed for the treatment of wide-necked intracranial cerebral aneurysms. The purpose of this study was to evaluate the safety, feasibility, and initial clinical results of using this device in patients.
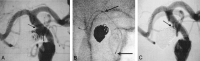
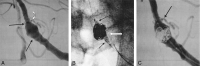
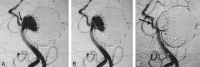
Methods: In clinical evaluation, five patients ranging in age from 54 to 71 years were electively treated. The smallest aneurysm measured 3.3 x 2.9 mm, and the largest aneurysm measured 10.6 x 8.5 mm (neck and height measurements).
Results: All five cases (100%) were technically successful without complications. In each case, the stent was accurately placed in the desired location, immediately followed by coil embolization to the desired degree of occlusion with a satisfactory result. The poststent and coil-occlusion angiogram demonstrated excellent blood flow across the stent, with satisfactory positioning of the coils within the aneurysm in all cases (100%). No patient suffered any clinical or neurologic complications, and all were discharged 1-3 days postprocedure, in stable condition with no new neurologic deficits.
Conclusion: In early clinical studies, the Cordis Enterprise stent performed well. The stent was able to be well visualized, deployed easily, could be repositioned if needed, and was accurately placed without technical difficulties. The closed cell design allowed all coils to be placed within the aneurysm and remain outside the flow of the parent artery. No periprocedural complications were encountered.
Figures

References
-
- Johnston SC, Wilson CB, Halbach VV, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol 2000;48:11–19 - PubMed
-
- Lempert TE, Malek AM, Halbach VV, et al. Endovascular treatment of ruptured posterior circulation cerebral aneurysms: clinical and angiographic outcomes. Stroke 2000;31:100–110 - PubMed
-
- Claiborne SC, Higashida RT, Barrow DL, et al. Recommendations for the endovascular treatment of intracranial aneurysms: a statement for healthcare professionals from the Committee on Cerebrovascular Imaging of the American Heart Association Council on Cardiovascular Radiology. Stroke 2002;33:2536–2544 - PubMed
-
- International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
