Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence
- PMID: 16093271
- PMCID: PMC4247030
- DOI: 10.1093/aob/mci215
Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence
Abstract
Background and aims: Akagare and Akiochi are diseases of rice associated with sulfide toxicity. This study investigates the possibility that rice reacts to sulfide by producing impermeable barriers in roots.
Methods: Root systems of rice, Oryza sativa cv. Norin 36, were subjected to short-term exposure to 0.174 mm sulfide (5.6 ppm) in stagnant solution. Root growth was monitored; root permeability was investigated in terms of polarographic determinations of oxygen efflux from fine laterals and the apices of adventitious roots, water uptake, anatomy and permeability to Fe2+ using potassium ferricyanide.
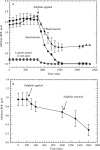
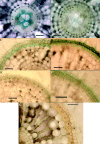
Key results: Both types of root responded rapidly to the sulfide with immediate cessation of growth, decreased radial oxygen loss (ROL) to the rhizospheres and reduced water uptake. Profiles of ROL measured from apex to basal regions of adventitious roots indicated that more intense barriers to ROL than normal were formed around the apices. Absorption of Fe2+ appeared to be impeded in sulfide-treated roots. In adventitious roots, deposition of lipid material (suberisation) and thickenings of walls within the superficial cell layers were obvious within a week after lifting the treatment and could prevent the emergence of laterals and commonly result in their upward longitudinal growth within the cortex. Death of laterals sometimes occurred prior to emergence; emergent laterals eventually died. In adventitious roots, blockages formed within the vascular and aeration systems in response to the sulfide.
Conclusions: In both adventitious and lateral roots, sulfide-induced cell wall suberization and thickening of the superficial layers were correlated with reduced permeability to O2, water and Fe2+. This study sheds light on some of the symptoms of diseases such as Akiochi. The results correlate with the authors' previous findings on the effects on roots of sulfide and lower organic acids in Phragmites and of acetic acid in rice.
Figures








References
-
- Allam AI. 1971.Soluble sulphides in rice fields and their in vitro effects on rice seedlings. PhD Dissertation, Louisiana State University, USA.
-
- Allam AI, Hollis JP. 1972. Sulphide inhibition of oxidases in rice roots. Phytopathology 62: 634–639.
-
- Allam AI, Pitts G, Hollis JP. 1972. Sulphide determination in soils with an ion-selective electrode. Soil Science 114: 457–467.
-
- Armstrong J. 1992.Pathways and mechanisms of aeration in Phragmites australis. PhD thesis, University of Hull, UK.
-
- Armstrong J, Armstrong W. 1999.Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytologist 142: 201–218.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

