Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice
- PMID: 16093309
- PMCID: PMC1189344
- DOI: 10.1073/pnas.0505579102
Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice
Abstract
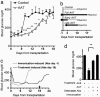
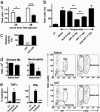
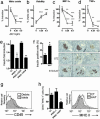
Islet transplantation for type 1 diabetic patients shows promising results with the use of nondiabetogenic immunosuppressive therapy. However, in addition to compromising the immune system of transplant recipients, long-term studies demonstrate that islet viability is impaired. Here, we demonstrate that, in the absence of immunosuppressive agents, monotherapy with clinical-grade human alpha1-antitrypsin (hAAT), the major serum serine-protease inhibitor, prolongs islet graft survival and normoglycemia in transplanted allogeneic diabetic mice, lasting until the development of anti-hAAT antibodies. Compared to untreated or albumin-control-treated graft recipients, which rejected islets at day 10, AAT-treated mice displayed diminished cellular infiltrates and intact intragraft insulin production throughout treatment. Using peritoneal infiltration models, we demonstrate that AAT decreases allogeneic fibroblast-elicited natural-killer-cell influx by 89%, CD3-positive cell influx by 44%, and thioglycolate-elicited neutrophil emigration by 66%. ATT also extended islet viability in mice after streptozotocin-induced beta cell toxicity. In vitro, several islet responses to IL-1beta/IFNgamma stimulation were examined. In the presence of AAT, islets displayed enhanced viability and inducible insulin secretion. Islets also released 36% less nitric oxide and 82% less macrophage inflammatory protein 1 alpha and expressed 63% fewer surface MHC class II molecules. TNFalpha release from IL-1beta/IFNgamma-stimulated islet cells was reduced by 99%, accompanied by an 8-fold increase in the accumulation of membrane TNFalpha on CD45-positive islet cells. In light of the established safety record and the nondiabetogenic potential of AAT, these data suggest that AAT may be beneficial as adjunctive therapy in patients undergoing islet transplantation.
Figures


Comment in
-
Saving islets from allograft rejection.Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12651-2. doi: 10.1073/pnas.0506079102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129823 Free PMC article. No abstract available.
Similar articles
-
α-1 Antitrypsin Enhances Islet Engraftment by Suppression of Instant Blood-Mediated Inflammatory Reaction.Diabetes. 2017 Apr;66(4):970-980. doi: 10.2337/db16-1036. Epub 2017 Jan 9. Diabetes. 2017. PMID: 28069642 Free PMC article.
-
alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice.Proc Natl Acad Sci U S A. 2008 Oct 21;105(42):16236-41. doi: 10.1073/pnas.0807627105. Epub 2008 Oct 13. Proc Natl Acad Sci U S A. 2008. PMID: 18852465 Free PMC article.
-
Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy.PLoS One. 2013 May 22;8(5):e63625. doi: 10.1371/journal.pone.0063625. Print 2013. PLoS One. 2013. PMID: 23717456 Free PMC article.
-
α1-antitrypsin increases interleukin-1 receptor antagonist production during pancreatic islet graft transplantation.Cell Mol Immunol. 2014 Jul;11(4):377-86. doi: 10.1038/cmi.2014.17. Epub 2014 Apr 7. Cell Mol Immunol. 2014. PMID: 25000533 Free PMC article.
-
Alpha-1 antitrypsin suppresses macrophage activation and promotes islet graft survival after intrahepatic islet transplantation.Am J Transplant. 2021 May;21(5):1713-1724. doi: 10.1111/ajt.16342. Epub 2020 Nov 16. Am J Transplant. 2021. PMID: 33047509 Free PMC article.
Cited by
-
Responses of IL-18- and IL-18 receptor-deficient pancreatic islets with convergence of positive and negative signals for the IL-18 receptor.Proc Natl Acad Sci U S A. 2006 Nov 7;103(45):16852-7. doi: 10.1073/pnas.0607917103. Epub 2006 Oct 30. Proc Natl Acad Sci U S A. 2006. PMID: 17075045 Free PMC article.
-
Alpha-1 Antitrypsin Therapy for Autoimmune Disorders.Chronic Obstr Pulm Dis. 2018 Oct 5;5(4):289-301. doi: 10.15326/jcopdf.5.4.2018.0131. Chronic Obstr Pulm Dis. 2018. PMID: 30723786 Free PMC article.
-
S-Nitrosylation of α1-Antitrypsin Triggers Macrophages Toward Inflammatory Phenotype and Enhances Intra-Cellular Bacteria Elimination.Front Immunol. 2019 Apr 2;10:590. doi: 10.3389/fimmu.2019.00590. eCollection 2019. Front Immunol. 2019. PMID: 31001247 Free PMC article.
-
α-1 Antitrypsin Enhances Islet Engraftment by Suppression of Instant Blood-Mediated Inflammatory Reaction.Diabetes. 2017 Apr;66(4):970-980. doi: 10.2337/db16-1036. Epub 2017 Jan 9. Diabetes. 2017. PMID: 28069642 Free PMC article.
-
Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation.J Clin Med. 2022 Jul 23;11(15):4290. doi: 10.3390/jcm11154290. J Clin Med. 2022. PMID: 35893379 Free PMC article.
References
-
- Sato, T., Inagaki, A., Uchida, K., Ueki, T., Goto, N., Matsuoka, S., Katayama, A., Haba, T., Tominaga, Y., Okajima, Y., et al. (2003) Transplantation 76, 1320-1326. - PubMed
-
- Ricordi, C. & Strom, T. B. (2004) Nat. Rev. Immunol. 4, 259-268. - PubMed
-
- Couzin, J. (2004) Science 306, 34-37. - PubMed
-
- Kobayashi, N., Okitsu, T., Lakey, J. R. & Tanaka, N. (2004) J. Artif. Organs 7, 1-8. - PubMed
-
- Breit, S. N., Wakefield, D., Robinson, J. P., Luckhurst, E., Clark, P. & Penny, R. (1985) Clin. Immunol. Immunopathol. 35, 363-380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

