Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis
- PMID: 16093341
- PMCID: PMC3062488
- DOI: 10.1152/jn.00351.2005
Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis
Abstract
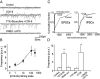
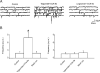
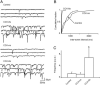
Cholecystokinin (CCK) is released from enteroendocrine cells after ingestion of nutrients and induces multiple effects along the gastrointestinal tract, including gastric relaxation and short-term satiety. We used whole cell patch-clamp and immunohistochemical techniques in rat brain stem slices to characterize the effects of CCK. In 45% of the neurons of nucleus tractus solitarius subnucleus centralis (cNTS), perfusion with the sulfated form of CCK (CCK-8s) increased the frequency of spontaneous excitatory currents (sEPSCs) in a concentration-dependent manner (1-300 nM). The threshold for the CCK-8s excitatory effect was 1 nM, the EC(50) was 20 nM, and E(max) was 100 nM. The excitatory effects of CCK-8s were still present when the slices were preincubated with tetrodotoxin or bicuculline or when the recordings were conducted with Cs(+) electrodes. Pretreatment with the CCK-A receptor antagonist, lorglumide (1 microM), antagonized the effects of CCK-8s, whereas perfusion with the CCK-B preferring agonist CCK-8 nonsulfated (CCK-ns, 1 microM) did not affect the frequency of sEPSCs. Similarly, pretreatment with the CCK-B receptor antagonist, triglumide (1 microM), did not prevent the actions of CCK-8s. Although the majority (i.e., 76%) of CCK-8s unresponsive cNTS neurons had a bipolar somata shape and were TH-IR negative, no differences were found in either the morphological or the neurochemical phenotype of cNTS neurons responsive to CCK-8s. Our results suggest that the excitatory effects of CCK-8s on terminals impinging on a subpopulation of cNTS neurons are mediated by CCK-A receptors; these responsive neurons, however, do not have morphological or neurochemical characteristics that automatically distinguish them from nonresponsive neurons.
Figures


References
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol. 1990;259:H1307–H1311. - PubMed
-
- Barraco R, El-Ridi M, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

