Myomodulin increases Ih and inhibits the NA/K pump to modulate bursting in leech heart interneurons
- PMID: 16093342
- PMCID: PMC1560091
- DOI: 10.1152/jn.00340.2005
Myomodulin increases Ih and inhibits the NA/K pump to modulate bursting in leech heart interneurons
Abstract
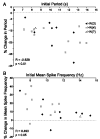
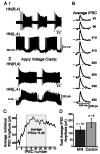
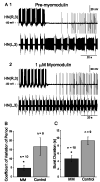
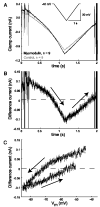
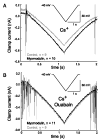
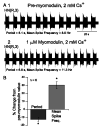
In the medicinal leech, a rhythmically active 14-interneuron network composes the central pattern generator for heartbeat. In two segmental ganglia, bilateral pairs of reciprocally inhibitory heart interneurons (oscillator interneurons) produce a rhythm of alternating bursts of action potentials that paces activity in the pattern-generating network. The neuropeptide myomodulin decreases the period of this bursting and increases the intraburst spike frequency when applied to isolated ganglia containing these oscillator interneurons. Myomodulin also decreases period, increases spike frequency, and increases the robustness of endogenous bursting in synaptically isolated (with bicuculline) oscillator interneurons. In voltage-clamp experiments using hyperpolarizing ramps, we identify an increase in membrane conductance elicited by myomodulin with the properties of a hyperpolarization-activated current. Voltage steps confirm that myomodulin indeed increases the maximum conductance of the hyperpolarization-activated current I(h). In similar experiments using Cs(+) to block I(h), we demonstrate that myomodulin also causes a steady offset in the ramp current that is not associated with an increase in conductance. This current offset is blocked by ouabain, indicating that myomodulin inhibits the Na/K pump. In current-clamp experiments, when I(h) is blocked with Cs(+), myomodulin decreases period and increases spike frequency of alternating bursting in synaptically connected oscillator interneurons, suggesting that inhibiting the Na/K pump modulates these burst characteristics. These observations indicate that myomodulin decreases period and increases spike frequency of endogenous bursting in synaptically isolated oscillator heart interneurons and alternating bursting of reciprocally inhibitory pairs of interneurons, at least in part, by increasing I(h) and by decreasing the Na/K pump.
Figures





Similar articles
-
Comodulation of h- and Na+/K+ Pump Currents Expands the Range of Functional Bursting in a Central Pattern Generator by Navigating between Dysfunctional Regimes.J Neurosci. 2021 Jul 28;41(30):6468-6483. doi: 10.1523/JNEUROSCI.0158-21.2021. Epub 2021 Jun 8. J Neurosci. 2021. PMID: 34103361 Free PMC article.
-
Hybrid systems analysis of the control of burst duration by low-voltage-activated calcium current in leech heart interneurons.J Neurophysiol. 2006 Dec;96(6):2857-67. doi: 10.1152/jn.00582.2006. Epub 2006 Aug 30. J Neurophysiol. 2006. PMID: 16943313
-
Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms.J Neurosci. 2002 Dec 15;22(24):10580-92. doi: 10.1523/JNEUROSCI.22-24-10580.2002. J Neurosci. 2002. PMID: 12486150 Free PMC article.
-
Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination, and modulation of rhythmic motor patterns.J Neurobiol. 1995 Jul;27(3):390-402. doi: 10.1002/neu.480270311. J Neurobiol. 1995. PMID: 7673897 Review.
-
Neural control of heartbeat in the leech and in some other invertebrates.Physiol Rev. 1979 Jan;59(1):101-36. doi: 10.1152/physrev.1979.59.1.101. Physiol Rev. 1979. PMID: 220645 Review.
Cited by
-
Low-threshold calcium currents contribute to locomotor-like activity in neonatal mice.J Neurophysiol. 2012 Jan;107(1):103-13. doi: 10.1152/jn.00583.2011. Epub 2011 Oct 12. J Neurophysiol. 2012. PMID: 21994264 Free PMC article.
-
A codimension-2 bifurcation controlling endogenous bursting activity and pulse-triggered responses of a neuron model.PLoS One. 2014 Jan 31;9(1):e85451. doi: 10.1371/journal.pone.0085451. eCollection 2014. PLoS One. 2014. PMID: 24497927 Free PMC article.
-
Bimodal modulation of short-term motor memory via dynamic sodium pumps in a vertebrate spinal cord.Curr Biol. 2022 Mar 14;32(5):1038-1048.e2. doi: 10.1016/j.cub.2022.01.012. Epub 2022 Jan 31. Curr Biol. 2022. PMID: 35104440 Free PMC article.
-
Control of Xenopus Tadpole Locomotion via Selective Expression of Ih in Excitatory Interneurons.Curr Biol. 2018 Dec 17;28(24):3911-3923.e2. doi: 10.1016/j.cub.2018.10.048. Epub 2018 Nov 29. Curr Biol. 2018. PMID: 30503615 Free PMC article.
-
The neuropeptide complement of the marine annelid Platynereis dumerilii.BMC Genomics. 2013 Dec 20;14:906. doi: 10.1186/1471-2164-14-906. BMC Genomics. 2013. PMID: 24359412 Free PMC article.
References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
-
- Angstadt JD, Friesen WO. Synchronized oscillatory activity in leech neurons induced by calcium channel blockers. J Neurophysiol. 1991;66:1858–1873. - PubMed
-
- Arbas EA. Rate modification in the heartbeat central pattern generator of the medicinal leech. J Comp Physiol A Sens Neural Behav Physiol. 1984;155:783–794.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

