Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion
- PMID: 16093349
- PMCID: PMC1237098
- DOI: 10.1091/mbc.e05-04-0310
Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion
Abstract
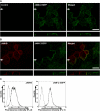
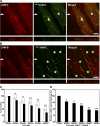
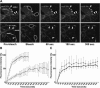
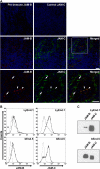
The junctional adhesion molecules (JAMs) have been recently described as interendothelial junctional molecules and as integrin ligands. Here we show that JAM-B and JAM-C undergo heterophilic interaction in cell-cell contacts and that JAM-C is recruited and stabilized in junctional complexes by JAM-B. In addition, soluble JAM-B dissociates soluble JAM-C homodimers to form JAM-B/JAM-C heterodimers. This suggests that the affinity of JAM-C monomers to form dimers is higher for JAM-B than for JAM-C. Using antibodies against JAM-C, the formation of JAM-B/JAM-C heterodimers can be abolished. This liberates JAM-C from its vascular binding partner JAM-B and makes it available on the apical side of vessels for interaction with its leukocyte counter-receptor alpha(M)beta2 integrin. We demonstrate that the modulation of JAM-C localization in junctional complexes is a new regulatory mechanism for alpha(M)beta2-dependent adhesion of leukocytes.
Figures


References
-
- Altamirano, M. M., Woolfson, A., Donda, A., Shamshiev, A., Briseno-Roa, L., Foster, N. W., Veprintsev, D. B., De Libero, G., Fersht, A. R., and Milstein, C. (2001). Ligand-independent assembly of recombinant human CD1 by using oxidative refolding chromatography. Proc. Natl. Acad. Sci. USA 98, 3288-3293. - PMC - PubMed
-
- Arrate, M. P., Rodriguez, J. M., Tran, T. M., Brock, T. A., and Cunningham, S. A. (2001). Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J. Biol. Chem. 276, 45826-45832. - PubMed
-
- Aurrand-Lions, M., Duncan, L., Ballestrem, C., and Imhof, B. A. (2001a). JAM-2, a novel Ig superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276, 2733-2741. - PubMed
-
- Aurrand-Lions, M., Johnson-Leger, C., Wong, C., Du Pasquier, L., and Imhof, B. A. (2001b). Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 98, 3699-3707. - PubMed
-
- Aurrand-Lions, M., Lamagna, C., Dangerfield, J. P., Wang, S., Herrera, P., Nourshargh, S., and Imhof, B. A. (2005). Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J. Immunol. 174, 6406-6415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

