Use-dependent inhibition of P2X3 receptors by nanomolar agonist
- PMID: 16093386
- PMCID: PMC6725291
- DOI: 10.1523/JNEUROSCI.5189-04.2005
Use-dependent inhibition of P2X3 receptors by nanomolar agonist
Abstract
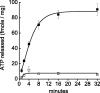
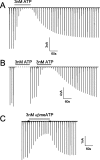
P2X3 receptors desensitize within 100 ms of channel activation, yet recovery from desensitization requires several minutes. The molecular basis for this slow rate of recovery is unknown. We designed experiments to test the hypothesis that this slow recovery is attributable to the high affinity (< 1 nM) of desensitized P2X3 receptors for agonist. We found that agonist binding to the desensitized state provided a mechanism for potent inhibition of P2X3 current. Sustained applications of 0.5 nM ATP inhibited > 50% of current to repetitive applications of P2X3 agonist. Inhibition occurred at 1000-fold lower agonist concentrations than required for channel activation and showed strong use dependence. No inhibition occurred without previous activation and desensitization. Our data are consistent with a model whereby inhibition of P2X3 by nanomolar [agonist] occurs by the rebinding of agonist to desensitized channels before recovery from desensitization. For several ATP analogs, the concentration required to inhibit P2X3 current inversely correlated with the rate of recovery from desensitization. This indicates that the affinity of the desensitized state and recovery rate primarily depend on the rate of agonist unbinding. Consistent with this hypothesis, unbinding of [32P]ATP from desensitized P2X3 receptors mirrored the rate of recovery from desensitization. As expected, disruption of agonist binding by site-directed mutagenesis increased the IC50 for inhibition and increased the rate of recovery.
Figures





References
-
- Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, van Biesen T (1999) Allosteric modulation and accelerated resensitization of human P2X(3) receptors by cibacron blue. J Pharmacol Exp Ther 291: 1135-1142. - PubMed
-
- Changeux JP (1990) The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol Sci 11: 485-492. - PubMed
-
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428-431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
