Dopamine is a regulator of arousal in the fruit fly
- PMID: 16093388
- PMCID: PMC6725300
- DOI: 10.1523/JNEUROSCI.2048-05.2005
Dopamine is a regulator of arousal in the fruit fly
Abstract
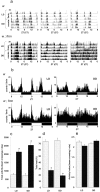
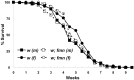
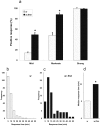
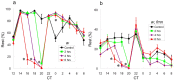
Sleep and arousal are known to be regulated by both homeostatic and circadian processes, but the underlying molecular mechanisms are not well understood. It has been reported that the Drosophila rest/activity cycle has features in common with the mammalian sleep/wake cycle, and it is expected that use of the fly genetic model will facilitate a molecular understanding of sleep and arousal. Here, we report the phenotypic characterization of a Drosophila rest/activity mutant known as fumin (fmn). We show that fmn mutants have abnormally high levels of activity and reduced rest (sleep); genetic mapping, molecular analyses, and phenotypic rescue experiments demonstrate that these phenotypes result from mutation of the Drosophila dopamine transporter gene. Consistent with the rest phenotype, fmn mutants show enhanced sensitivity to mechanical stimuli and a prolonged arousal once active, indicating a decreased arousal threshold. Strikingly,fmn mutants do not show significant rebound in response to rest deprivation as is typical for wild-type flies, nor do they show decreased life span. These results provide direct evidence that dopaminergic signaling has a critical function in the regulation of insect arousal.
Figures

References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, et al. (2000) The genome sequence of Drosophila melanogaster Science 287: 2185-2195. - PubMed
-
- Andretic R, Shaw PJ (2005) Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol 393: 759-772. - PubMed
-
- Andretic R, Chaney S, Hirsh J (1999) Circadian genes are required for cocaine sensitization in Drosophila Science 285: 1066-1068. - PubMed
-
- Andretic R, van Swinderen B, Greenspan RJ (2005) Dopaminergic modulation of arousal in Drosophila Curr Biol 15: 1165-1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
