Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus
- PMID: 16093392
- PMCID: PMC6725307
- DOI: 10.1523/JNEUROSCI.1008-05.2005
Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus
Abstract
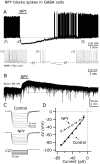
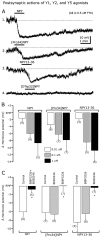
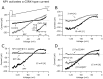
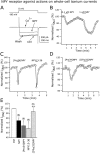
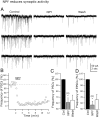
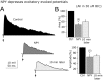
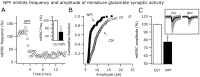
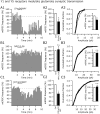
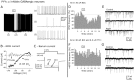
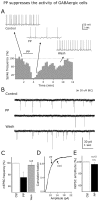
The fast inhibitory transmitter GABA is robustly expressed in the arcuate nucleus (ARC) and appears to play a major role in hypothalamic regulation of endocrine function and energy homeostasis. Previously, it has not been possible to record selectively from GABA cells, because they have no defining morphological or physiological characteristics. Using transgenic mice that selectively express GFP (green fluorescent protein) in GAD67 (glutamic acid decarboxylase 67)-synthesizing cells, we identified ARC GABA neurons (n > 300) and used whole-cell recording to study their physiological response to neuropeptide Y (NPY), the related peptide YY(3-36) (PYY(3-36)), and pancreatic polypeptide (PP), important modulators of ARC function. In contrast to other identified ARC cells in which NPY receptor agonists were reported to generate excitatory actions, we found that NPY consistently reduced the firing rate and hyperpolarized GABA neurons including neuroendocrine GABA neurons identified by antidromic median eminence stimulation. The inhibitory NPY actions were mediated by postsynaptic activation of G-protein-linked inwardly rectifying potassium (GIRK) and depression of voltage-gated calcium currents via Y1 and Y2 receptor subtypes. Additionally, NPY reduced spontaneous and evoked synaptic glutamate release onto GABA neurons by activation of Y1 and Y5 receptors. The peptide PYY(3-36), a peripheral endocrine signal that can act in the brain, also inhibited GABA neurons, including identified neuroendocrine cells, by activating GIRK conductances and depressing calcium currents. The endogenous Y4 agonist PP depressed the activity of GABA-expressing neurons mainly by presynaptic attenuation of glutamate release. Together, these results show that the family of neuropeptide Y modulators reduces the activity of inhibitory GABA neurons in the ARC by multiple presynaptic and postsynaptic mechanisms.
Figures



References
-
- Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P (1992) Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse, and guinea pig. J Comp Neurol 323: 103-116. - PubMed
-
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M (1985) An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res 331: 172-175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
