Properties of motility in Bacillus subtilis powered by the H+-coupled MotAB flagellar stator, Na+-coupled MotPS or hybrid stators MotAS or MotPB
- PMID: 16095621
- PMCID: PMC2578835
- DOI: 10.1016/j.jmb.2005.07.030
Properties of motility in Bacillus subtilis powered by the H+-coupled MotAB flagellar stator, Na+-coupled MotPS or hybrid stators MotAS or MotPB
Abstract
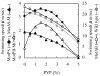
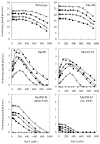
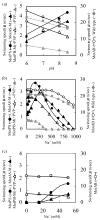
Bacillus subtilis has a single set of flagellar rotor proteins that interact with two distinct stator-force generators, the H+-coupled MotAB complex and the Na+-coupled MotPS complex, that energize rotation. Here, motility on soft agar plates and in liquid was assayed in wild-type B.subtilis and strains expressing only one stator, either MotAB, MotPS or hybrid MotAS or MotPB. The strains expressing MotAB or MotAS had an average of 11 flagella/cell while those expressing MotPS or MotPB had an average of seven flagella/cell, and a Mot-less double mutant had three to four flagella/cell. MotAB had a more dominant role in motility than MotPS under most conditions, but MotPS supported comparable motility to MotAB on malate-containing soft agar plating media at elevated pH and Na+. MotAB supported much faster swimming speeds in liquid than MotPS, MotAS or MotPB under all conditions, but a contribution of MotPS to wild-type swimming was discernible from differences in swimming speeds of wild-type and MotAB at elevated viscosity, pH and Na+. Swimming supported by MotPS and MotAS was stimulated by Na+ and elevated pH whereas the converse was true of MotAB and MotPB. This suggests that MotAS is Na+-coupled and MotPB is H+-coupled and that MotB and MotS are major determinants of ion-coupling. However, the swimming speed supported by MotPB, as well as MotPS and MotAS, was inhibited severely at Na+ concentrations above 300 mM whereas MotAB-dependent swimming was not. The presence of either the MotP or MotS component in the stator also conferred sensitivity to inhibition by an amiloride analogue. These observations suggest that MotP contributes to Na+-coupling and inhibition by Na+ channel inhibitors. Similarly, a role for MotA in H+-dependent stator properties is indicated by the larger effects of pH on the Na+-response of MotAS versus MotPS. Finally, optimal function at elevated viscosity was found only in MotPS and MotPB and is therefore conferred by MotP.
Figures
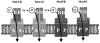

Similar articles
-
Motility and chemotaxis in alkaliphilic Bacillus species.Future Microbiol. 2009 Nov;4(9):1137-49. doi: 10.2217/fmb.09.76. Future Microbiol. 2009. PMID: 19895217 Free PMC article. Review.
-
Mutations alter the sodium versus proton use of a Bacillus clausii flagellar motor and confer dual ion use on Bacillus subtilis motors.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14359-64. doi: 10.1073/pnas.0802106105. Epub 2008 Sep 16. Proc Natl Acad Sci U S A. 2008. PMID: 18796609 Free PMC article.
-
Mutational analysis of charged residues in the cytoplasmic loops of MotA and MotP in the Bacillus subtilis flagellar motor.J Biochem. 2014 Oct;156(4):211-20. doi: 10.1093/jb/mvu030. Epub 2014 Apr 26. J Biochem. 2014. PMID: 24771657
-
MotPS is the stator-force generator for motility of alkaliphilic Bacillus, and its homologue is a second functional Mot in Bacillus subtilis.Mol Microbiol. 2004 Aug;53(4):1035-49. doi: 10.1111/j.1365-2958.2004.04173.x. Mol Microbiol. 2004. PMID: 15306009
-
Dynamic exchange of two types of stator units in Bacillus subtilis flagellar motor in response to environmental changes.Comput Struct Biotechnol J. 2020 Oct 15;18:2897-2907. doi: 10.1016/j.csbj.2020.10.009. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33163150 Free PMC article. Review.
Cited by
-
Evolution of the Stator Elements of Rotary Prokaryote Motors.J Bacteriol. 2020 Jan 15;202(3):e00557-19. doi: 10.1128/JB.00557-19. Print 2020 Jan 15. J Bacteriol. 2020. PMID: 31591272 Free PMC article. Review.
-
Dynamic Hybrid Flagellar Motors-Fuel Switch and More.Front Microbiol. 2022 Apr 12;13:863804. doi: 10.3389/fmicb.2022.863804. eCollection 2022. Front Microbiol. 2022. PMID: 35495728 Free PMC article. Review.
-
Pulcherrimin protects Bacillus subtilis against oxidative stress during biofilm development.NPJ Biofilms Microbiomes. 2023 Jul 19;9(1):50. doi: 10.1038/s41522-023-00418-z. NPJ Biofilms Microbiomes. 2023. PMID: 37468524 Free PMC article.
-
Spirochete Flagella and Motility.Biomolecules. 2020 Apr 4;10(4):550. doi: 10.3390/biom10040550. Biomolecules. 2020. PMID: 32260454 Free PMC article. Review.
-
Motility and chemotaxis in alkaliphilic Bacillus species.Future Microbiol. 2009 Nov;4(9):1137-49. doi: 10.2217/fmb.09.76. Future Microbiol. 2009. PMID: 19895217 Free PMC article. Review.
References
-
- Berg HC, Anderson RA. Bacteria swim by rotating their flagellar filaments. Nature. 1973;245:380–382. - PubMed
-
- Kojima S, Blair DF. The bacterial flagellar motor: structure and function of a complex molecular machine. Int Rev Cytol. 2004;233:93–134. - PubMed
-
- Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

