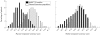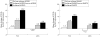Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis
- PMID: 16096330
- PMCID: PMC1798094
- DOI: 10.1136/ard.2005.040196
Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis
Abstract
Objectives: To evaluate the effect of infliximab on health related quality of life (HRQoL) and physical function in patients with active psoriatic arthritis (PsA) in the IMPACT 2 trial.
Methods: 200 patients with PsA unresponsive to conventional treatment were randomised to intravenous infusions of infliximab 5 mg/kg or placebo at weeks 0, 2, 6, 14, and 22; patients with inadequate response entered early escape at week 16. HRQoL was assessed using the Short Form-36 (SF-36) at weeks 0, 14, and 24. Functional disability was assessed using the Health Assessment Questionnaire (HAQ) at every visit through week 24. Associations between changes in quality of life (SF-36) and articular (American College of Rheumatology (ACR)) and dermatological (Psoriasis Area and Severity Index (PASI)) responses were examined.
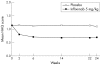
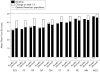
Results: Mean percentage improvement from baseline in HAQ was 48.6% in the infliximab group compared with worsening of 18.4% in the placebo group at week 14 (p < 0.001). Furthermore, 58.6% and 19.4% of infliximab and placebo treated patients, respectively, achieved a clinically meaningful improvement in HAQ (that is, > or = 0.3 unit decrease) at week 14 (p < 0.001). Increases in physical and mental component summary (PCS and MCS) scores and all eight scales of the SF-36 in the infliximab group were greater than those in the placebo group at week 14 (p < or = 0.001). These benefits were sustained through week 24. Patients achieving ACR20 and PASI75 responses had the greatest improvements in PCS and MCS scores.
Conclusions: In patients with PsA, infliximab 5 mg/kg significantly improved HRQoL and physical function compared with placebo through 24 weeks.
References
-
- Gladman D D, Shuckett R, Russell M L, Thorne J C, Schachter R K. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med 198762127–141. - PubMed
-
- Torre Alonso J C, Rodriguez Perez A, Arribas Castrillo J M, Ballina Garcia J, Riestra Noriega J L, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunologic and radiological study of 180 patients. Br J Rheumatol 199130245–250. - PubMed
-
- Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003421460–1468. - PubMed
-
- Rahman P, Nguyen E, Cheung C, Schentag C T, Gladman D D. Comparison of radiological severity in psoriatic arthritis and rheumatoid arthritis. J Rheumatol 2001281041–1044. - PubMed
-
- Sokoll K B, Helliwell P S. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001281842–1846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous