Acetylation of HIV-1 integrase by p300 regulates viral integration
- PMID: 16096645
- PMCID: PMC1201351
- DOI: 10.1038/sj.emboj.7600770
Acetylation of HIV-1 integrase by p300 regulates viral integration
Abstract
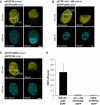
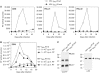
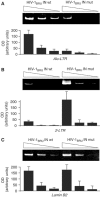
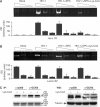
Integration of HIV-1 into the human genome, which is catalyzed by the viral protein integrase (IN), preferentially occurs near transcriptionally active genes. Here we show that p300, a cellular acetyltransferase that regulates chromatin conformation through the acetylation of histones, also acetylates IN and controls its activity. We have found that p300 directly binds IN both in vitro and in the cells, as also specifically demonstrated by fluorescence resonance energy transfer technique analysis. This interaction results in the acetylation of three specific lysines (K264, K266, K273) in the carboxy-terminus of IN, a region that is required for DNA binding. Acetylation increases IN affinity to DNA, and promotes the DNA strand transfer activity of the protein. In the context of the viral replication cycle, point mutations in the IN acetylation sites abolish virus replication by specifically impairing its integration capacity. This is the first demonstration that HIV-1 IN activity is specifically regulated by post-translational modification.
Figures




References
-
- Bandyopadhyay D, Okan NA, Bales E, Nascimento L, Cole PA, Medrano EE (2002) Down-regulation of p300/CBP histone acetyltransferase activates a senescence checkpoint in human melanocytes. Cancer Res 62: 6231–6239 - PubMed
-
- Cara A, Guarnaccia F, Reitz MS, Gallo RC, Lori F (1995) Self-limiting, cell type-dependent replication of an integrase-defective human immunodeficiency virus type 1 in human primary macrophages but not T lymphocytes. Virology 208: 242–248 - PubMed
-
- Carrozza MJ, Utley RT, Workman JL, Cote J (2003) The diverse functions of histone acetyltransferase complexes. Trends Genet 19: 321–329 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

