The U11/U12 snRNP 65K protein acts as a molecular bridge, binding the U12 snRNA and U11-59K protein
- PMID: 16096647
- PMCID: PMC1201347
- DOI: 10.1038/sj.emboj.7600765
The U11/U12 snRNP 65K protein acts as a molecular bridge, binding the U12 snRNA and U11-59K protein
Abstract
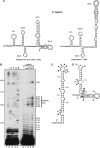
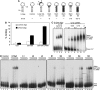
U11 and U12 interact cooperatively with the 5' splice site and branch site of pre-mRNA as a stable preformed di-snRNP complex, thereby bridging the 5' and 3' ends of the intron within the U12-dependent prespliceosome. To identify proteins contributing to di-snRNP formation and intron bridging, we investigated protein-protein and protein-RNA interactions between components of the U11/U12 snRNP. We demonstrate that the U11/U12-65K protein possesses dual binding activity, interacting directly with U12 snRNA via its C-terminal RRM and the U11-associated 59K protein via its N-terminal half. We provide evidence that, in contrast to the previously published U12 snRNA secondary structure model, the 3' half of U12 forms an extended stem-loop with a highly conserved seven-nucleotide loop and that the latter serves as the 65K binding site. Addition of an oligonucleotide comprising the 65K binding site to an in vitro splicing reaction inhibited U12-dependent, but not U2-dependent, pre-mRNA splicing. Taken together, these data suggest that U11/U12-65K and U11-59K contribute to di-snRNP formation and intron bridging in the minor prespliceosome.
Figures


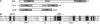
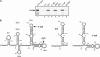


References
-
- Achsel T, Ahrens K, Brahms H, Teigelkamp S, Lührmann R (1998) The human U5-220kD protein (hPrp8) forms a stable RNA-free complex with several U5-specific proteins, including an RNA unwindase, a homologue of ribosomal elongation factor EF-2, and a novel WD-40 protein. Mol Cell Biol 18: 6756–6766 - PMC - PubMed
-
- Bandziulis RJ, Swanson MS, Dreyfuss G (1989) RNA-binding proteins as developmental regulators. Genes Dev 3: 431–437 - PubMed
-
- Brunel C, Romby P (2000) Probing RNA structure and RNA–ligand complexes with chemical probes. Methods Enzymol 318: 3–21 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

