Induction of apoptosis by lipophilic activators of CTP:phosphocholine cytidylyltransferase alpha (CCTalpha)
- PMID: 16097951
- PMCID: PMC1316283
- DOI: 10.1042/BJ20051021
Induction of apoptosis by lipophilic activators of CTP:phosphocholine cytidylyltransferase alpha (CCTalpha)
Abstract
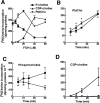
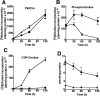
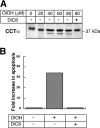
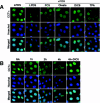
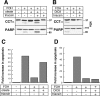
Farnesol (FOH) inhibits the CDP-choline pathway for PtdCho (phosphatidylcholine) synthesis, an activity that is involved in subsequent induction of apoptosis. Interestingly, the rate-limiting enzyme in this pathway, CCTalpha (CTP:phosphocholine cytidylyltransferase alpha), is rapidly activated, cleaved by caspases and exported from the nucleus during FOH-induced apoptosis. The purpose of the present study was to determine how CCTalpha activity and PtdCho synthesis contributed to induction of apoptosis by FOH and oleyl alcohol. Contrary to previous reports, we show that the initial effect of FOH and oleyl alcohol was a rapid (10-30 min) and transient activation of PtdCho synthesis. During this period, the mass of DAG (diacylglycerol) decreased by 40%, indicating that subsequent CDP-choline accumulation and inhibition of PtdCho synthesis could be due to substrate depletion. At later time points (>1 h), FOH and oleyl alcohol promoted caspase cleavage and nuclear export of CCTalpha, which was prevented by treatment with oleate or DiC8 (dioctanoylglycerol). Protection from FOH-induced apoptosis required CCTalpha activity and PtdCho synthesis since (i) DiC8 and oleate restored PtdCho synthesis, but not endogenous DAG levels, and (ii) partial resistance was conferred by stable overexpression of CCTalpha and increased PtdCho synthesis in CCTalpha-deficient MT58 cells. These results show that DAG depletion by FOH or oleyl alcohol could be involved in inhibition of PtdCho synthesis. However, decreased DAG was not sufficient to induce apoptosis provided nuclear CCTalpha and PtdCho syntheses were sustained.
Figures
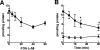

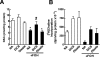
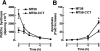
Similar articles
-
Oxysterol activation of phosphatidylcholine synthesis involves CTP:phosphocholine cytidylyltransferase alpha translocation to the nuclear envelope.Biochem J. 2009 Feb 15;418(1):209-17. doi: 10.1042/BJ20081923. Biochem J. 2009. PMID: 18980580
-
Caspase processing and nuclear export of CTP:phosphocholine cytidylyltransferase alpha during farnesol-induced apoptosis.Mol Cell Biol. 2002 Jul;22(13):4851-62. doi: 10.1128/MCB.22.13.4851-4862.2002. Mol Cell Biol. 2002. PMID: 12052891 Free PMC article.
-
Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain.J Lipid Res. 2009 May;50(5):966-76. doi: 10.1194/jlr.M800632-JLR200. Epub 2008 Dec 20. J Lipid Res. 2009. PMID: 19098306 Free PMC article.
-
Phosphatidylcholine and the CDP-choline cycle.Biochim Biophys Acta. 2013 Mar;1831(3):523-32. doi: 10.1016/j.bbalip.2012.09.009. Epub 2012 Sep 23. Biochim Biophys Acta. 2013. PMID: 23010477 Free PMC article. Review.
-
CTP:phosphocholine cytidylyltransferase: Function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis.Prog Lipid Res. 2015 Jul;59:147-71. doi: 10.1016/j.plipres.2015.07.001. Epub 2015 Jul 9. Prog Lipid Res. 2015. PMID: 26165797 Review.
Cited by
-
ras-Induced up-regulation of CTP:phosphocholine cytidylyltransferase α contributes to malignant transformation of intestinal epithelial cells.J Biol Chem. 2013 Jan 4;288(1):633-43. doi: 10.1074/jbc.M112.347682. Epub 2012 Nov 15. J Biol Chem. 2013. PMID: 23155050 Free PMC article.
-
A mechanism for suppression of the CDP-choline pathway during apoptosis.J Lipid Res. 2013 Dec;54(12):3373-84. doi: 10.1194/jlr.M041434. Epub 2013 Oct 17. J Lipid Res. 2013. PMID: 24136823 Free PMC article.
-
Choline phosphate cytidylyltransferase-α is a novel antigen detected by the anti-ERCC1 antibody 8F1 with biomarker value in patients with lung and head and neck squamous cell carcinomas.Cancer. 2014 Jun 15;120(12):1898-907. doi: 10.1002/cncr.28643. Epub 2014 Apr 1. Cancer. 2014. PMID: 24692084 Free PMC article.
-
An Approach to Minimize Tumour Proliferation by Reducing the Formation of Components for Cell Membrane.Molecules. 2022 Apr 24;27(9):2735. doi: 10.3390/molecules27092735. Molecules. 2022. PMID: 35566086 Free PMC article.
-
CCT α is a novel biomarker for diagnosis of laryngeal squamous cell cancer.Sci Rep. 2019 Aug 14;9(1):11823. doi: 10.1038/s41598-019-47895-x. Sci Rep. 2019. PMID: 31413263 Free PMC article.
References
-
- Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 1994;269:3858–3867. - PubMed
-
- Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. J. Biol. Chem. 1996;271:20219–20222. - PubMed
-
- Cui Z., Houweling M., Chen M. H., Record M., Chap H., Vance D. E., Terce F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 1996;271:14668–14671. - PubMed
-
- Rodriguez-Gonzalez A., de Molina A. R., Fernandez F., Ramos M. A., Nunez Mdel C., Campos J., Lacal J. C. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene. 2003;22:8803–8812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

