The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism
- PMID: 16098191
- PMCID: PMC2234577
- DOI: 10.1111/j.1742-4658.2005.04819.x
The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism
Abstract
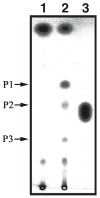
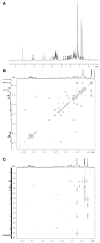
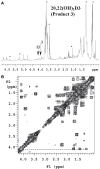
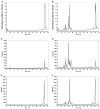
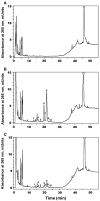
We show that cytochrome P450scc (CYP11A1) in either a reconstituted system or in isolated adrenal mitochondria can metabolize vitamin D3. The major products of the reaction with reconstituted enzyme were 20-hydroxycholecalciferol and 20,22-dihydroxycholecalciferol, with yields of 16 and 4%, respectively, of the original vitamin D3 substrate. Trihydroxycholecalciferol was a minor product, likely arising from further metabolism of dihydroxycholecalciferol. Based on NMR analysis and known properties of P450scc we propose that hydroxylation of vitamin D3 by P450scc occurs sequentially and stereospecifically with initial formation of 20(S)-hydroxyvitamin D3. P450scc did not metabolize 25-hydroxyvitamin D3, indicating that modification of C25 protected it against P450scc action. Adrenal mitochondria also metabolized vitamin D3 yielding 10 hydroxyderivatives, with UV spectra typical of vitamin D triene chromophores. Aminogluthimide inhibition showed that the three major metabolites, but not the others, resulted from P450scc action. It therefore appears that non-P450scc enzymes present in the adrenal cortex to some extent contribute to metabolism of vitamin D3. We conclude that purified P450scc in a reconstituted system or P450scc in adrenal mitochondria can add one hydroxyl group to vitamin D3 with subsequent hydroxylation being observed for reconstituted enzyme but not for adrenal mitochondria. Additional vitamin D3 metabolites arise from the action of other enzymes in adrenal mitochondria. These findings appear to define novel metabolic pathways involving vitamin D3 that remain to be characterized.
Figures


References
-
- Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. - PubMed
-
- Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. - PubMed
-
- Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. - PubMed
-
- Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–288. - PubMed
-
- Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

