Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma
- PMID: 16098465
- PMCID: PMC3024718
- DOI: 10.1016/j.ccr.2005.07.004
Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma
Abstract
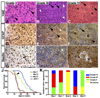
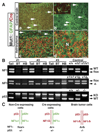
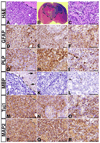
Malignant astrocytoma, the most prevalent primary brain tumor, is resistant to all known therapies and frequently harbors mutations that inactivate p53 and activate Ras signaling. We have generated mouse strains that lack p53 and harbor a conditional allele of the NF1 tumor suppressor that negatively regulates Ras signaling. The mice develop malignant astrocytomas with complete penetrance. The majority of tumors display characteristics of glioblastoma multiforme with concomitant alteration of signaling pathways previously described in the human counterparts of this neoplasm. We find that the sequence of tumor suppressor inactivation influences tumorigenicity and that earliest evidence of tumor formation localizes to regions of the brain that contain a multipotent stem cell population capable of in vivo differentiation into neurons and glia.
Figures





References
-
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
-
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. - PubMed
-
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

