Analysis of thrombocyte development in CD41-GFP transgenic zebrafish
- PMID: 16099879
- PMCID: PMC1895094
- DOI: 10.1182/blood-2005-01-0179
Analysis of thrombocyte development in CD41-GFP transgenic zebrafish
Abstract
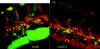
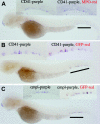
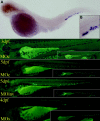
Thrombocytes are the nucleated equivalent of platelets in nonmammalian vertebrates such as the zebrafish, Danio rerio. We have cloned zebrafish CD41 cDNA (alpha(IIb), glycoprotein IIb [GPIIb]) and its promoter and have generated transgenic zebrafish lines with green fluorescent protein (GFP)-tagged thrombocytes. CD41 mRNA transcripts appeared 42 hours after fertilization (hpf) by reverse-transcriptase-polymerase chain reaction (RT-PCR) and at 48 hpf in circulating hematopoietic cells. Flow sorting of thrombocytes from the mesonephros of adult CD41-GFP zebrafish showed a GFP(high) subset, which had the morphologic appearance of mature thrombocytes, and a GFP(low) subset with an immature appearance, suggesting that they may be thrombocyte precursors. Confocal laser microscopy of embryos 40 and 48 hpf also showed a nonmobile population of GFP+ cells in a discrete area between the dorsal aorta and caudal vein. Production of circulating thrombocytes was inhibited by the injection of antisense morpholinos for the stem-cell transcription factor scl and c-mpl, the receptor for thrombopoietin. The nonmobile pool of GFP+ cells was abolished by scl knockdown and partially inhibited by c-mpl knockdown. These studies have shown that it is possible to identify thrombocytes, thrombocyte precursors, and, possibly, early hematopoietic stem cells in zebrafish embryos and track their proliferation and maturation.
Figures




References
-
- Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107: 731-738. - PubMed
-
- Hartwig J, Italiano J Jr. The birth of the platelet. J Thromb Haemost. 2003;1: 1580-1586. - PubMed
-
- Poncz M, Surrey S, LaRocco P, et al. Cloning and characterization of platelet factor 4 cDNA derived from a human erythroleukemic cell line. Blood. 1987;69: 219-223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

