Phase behavior and nanoscale structure of phospholipid membranes incorporated with acylated C14-peptides
- PMID: 16100273
- PMCID: PMC1366748
- DOI: 10.1529/biophysj.105.060756
Phase behavior and nanoscale structure of phospholipid membranes incorporated with acylated C14-peptides
Abstract
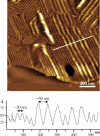
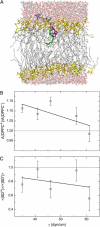
The thermotropic phase behavior and lateral structure of dipalmitoylphosphatidylcholine (DPPC) lipid bilayers containing an acylated peptide has been characterized by differential scanning calorimetry (DSC) on vesicles and atomic force microscopy (AFM) on mica-supported bilayers. The acylated peptide, which is a synthetic decapeptide N-terminally linked to a C14 acyl chain (C14-peptide), is incorporated into DPPC bilayers in amounts ranging from 0-20 mol %. The calorimetric scans of the two-component system demonstrate a distinct influence of the C14-peptide on the lipid bilayer thermodynamics. This is manifested as a concentration-dependent downshift of both the main phase transition and the pretransition. In addition, the main phase transition peak is significantly broadened, indicating phase coexistence. In the AFM imaging scans we found that the C14-peptide, when added to supported gel phase DPPC bilayers, inserts preferentially into preexisting defect regions and has a noticeable influence on the organization of the surrounding lipids. The presence of the C14-peptide gives rise to a laterally heterogeneous bilayer structure with coexisting lipid domains characterized by a 10 A height difference. The AFM images also show that the appearance of the ripple phase of the DPPC lipid bilayers is unaffected by the C14-peptide. The experimental results are supported by molecular dynamics simulations, which show that the C14-peptide has a disordering effect on the lipid acyl chains and causes a lateral expansion of the lipid bilayer. These effects are most pronounced for gel-like bilayer structures and support the observed downshift in the phase-transition temperature. Moreover, the molecular dynamics data indicate a tendency of a tryptophan residue in the peptide sequence to position itself in the bilayer headgroup region.
Figures




Similar articles
-
A DSC and FTIR spectroscopic study of the effects of the epimeric 4-cholesten-3-ols and 4-cholesten-3-one on the thermotropic phase behaviour and organization of dipalmitoylphosphatidylcholine bilayer membranes: comparison with their 5-cholesten analogues.Chem Phys Lipids. 2014 Jan;177:71-90. doi: 10.1016/j.chemphyslip.2013.11.008. Epub 2013 Dec 1. Chem Phys Lipids. 2014. PMID: 24296232
-
A calorimetric and spectroscopic comparison of the effects of lathosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes.Biochemistry. 2011 Nov 22;50(46):9982-97. doi: 10.1021/bi200721j. Epub 2011 Oct 26. Biochemistry. 2011. PMID: 21951051
-
A DSC and FTIR spectroscopic study of the effects of the epimeric cholestan-3-ols and cholestan-3-one on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes: Comparison with their 5-cholesten analogs.Chem Phys Lipids. 2015 Apr;187:34-49. doi: 10.1016/j.chemphyslip.2015.02.002. Epub 2015 Feb 27. Chem Phys Lipids. 2015. PMID: 25732198
-
Atomistic Monte Carlo simulation of lipid membranes.Int J Mol Sci. 2014 Jan 24;15(2):1767-803. doi: 10.3390/ijms15021767. Int J Mol Sci. 2014. PMID: 24469314 Free PMC article. Review.
-
Lipid bilayers: thermodynamics, structure, fluctuations, and interactions.Chem Phys Lipids. 2004 Jan;127(1):3-14. doi: 10.1016/j.chemphyslip.2003.09.002. Chem Phys Lipids. 2004. PMID: 14706737 Free PMC article. Review.
Cited by
-
Effects of palmitoylation on dynamics and phospholipid-bilayer-perturbing properties of the N-terminal segment of pulmonary surfactant protein SP-C as shown by 2H-NMR.Biophys J. 2008 Sep;95(5):2308-17. doi: 10.1529/biophysj.108.132845. Epub 2008 May 23. Biophys J. 2008. PMID: 18502795 Free PMC article.
-
Interactions of poly(amidoamine) dendrimers with Survanta lung surfactant: the importance of lipid domains.Langmuir. 2008 Oct 7;24(19):11003-8. doi: 10.1021/la801497d. Epub 2008 Sep 3. Langmuir. 2008. PMID: 18763817 Free PMC article.
-
Biophysical approaches for exploring lipopeptide-lipid interactions.Biochimie. 2020 Mar;170:173-202. doi: 10.1016/j.biochi.2020.01.009. Epub 2020 Jan 21. Biochimie. 2020. PMID: 31978418 Free PMC article. Review.
-
Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study.Biomolecules. 2025 Mar 26;15(4):486. doi: 10.3390/biom15040486. Biomolecules. 2025. PMID: 40305216 Free PMC article.
-
Biomembrane sensitivity to structural changes in bound polymers.J Am Chem Soc. 2009 Feb 11;131(5):1666-7. doi: 10.1021/ja808461s. J Am Chem Soc. 2009. PMID: 19152326 Free PMC article.
References
-
- Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1451:1–16. - PubMed
-
- Silvius, J. R. 1999. Lipid modifications of intracellular signal-transducing proteins. J. Liposome Res. 9:1–19.
-
- Mouritsen, O. G. (2005). Life—As a Matter of Fat. The Emerging Science of Lipidomics. Springer-Verlag, Heidelberg, Germany.
-
- McLaughlin, A., and A. Aderem. 1995. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem. Sci. 20:272–276. - PubMed
-
- Dell'aqua, M. L., and J. D. Scott. 1997. Protein kinase A anchoring. J. Biol. Chem. 272:12881–12884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

