I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow
- PMID: 16100282
- PMCID: PMC1366851
- DOI: 10.1529/biophysj.104.057729
I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow
Erratum in
- Biophys J. 2007 Jan 15;92(2):696
Abstract
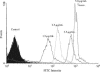
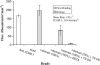
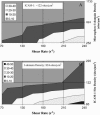
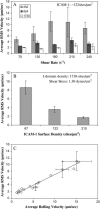
In their active state, beta(2)-integrins, such as LFA-1, mediate the firm arrest of leukocytes by binding intercellular adhesion molecules (ICAMs) expressed on endothelium. Although the primary function of LFA-1 is assumed to be the ability to mediate firm adhesion, recent work has shown that LFA-1 can contribute to cell tethering and rolling under hydrodynamic flow, a role previously largely attributed to the selectins. The inserted (I) domain of LFA-1 has recently been crystallized in the wild-type (wt) and locked-open conformations and has been shown to, respectively, support rolling and firm adhesion under flow when expressed in alpha(L)beta(2) heterodimers or as isolated domains on cells. Here, we report results from cell-free adhesion assays where wt I-domain-coated polystyrene particles were allowed to interact with ICAM-1-coated surfaces in shear flow. We show that wt I-domain can independently mediate the capture of particles from flow and support their rolling on ICAM-1 surfaces in a manner similar to how carbohydrate-selectin interactions mediate rolling. Adhesion is specific and blocked by appropriate antibodies. We also show that the rolling velocity of I-domain-coated particles depends on the wall shear stress in flow chamber, I-domain site density on microsphere surfaces, and ICAM-1 site density on substrate surfaces. Furthermore, we show that rolling is less sensitive to wall shear stress and ICAM-1 substrate density at high density of I-domain on the microsphere surface. Computer simulations using adhesive dynamics can recreate bead rolling dynamics and show that the mechanochemical properties of ICAM-1-I-domain interactions are similar to those of carbohydrate-selectin interactions. Understanding the biophysics of adhesion mediated by the I-domain of LFA-1 can elucidate the complex roles this integrin plays in leukocyte adhesion in inflammation.
Figures



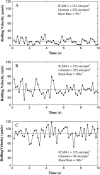
References
-
- Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. - PubMed
-
- Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 65:859–873. - PubMed
-
- Tonnesen, M. G. 1989. Neutrophil-endothelial cell interactions: mechanisms of neutrophil adherence to vascular endothelium. J. Invest. Dermatol. 93:53S–58S. - PubMed
-
- Gopalan, P. K., C. W. Smith, H. Lu, E. L. Berg, L. V. McIntire, and S. I. Simon. 1997. Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin. J. Immunol. 158:367–375. - PubMed
-
- Stewart, M. P., C. Cabanas, and N. Hogg. 1996. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J. Immunol. 156:1810–1817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

