Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia
- PMID: 16100289
- PMCID: PMC2661116
- DOI: 10.1152/ajplung.00133.2005
Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia
Abstract
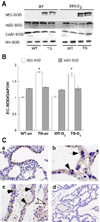
Transgenic (TG) human (h) extracellular superoxide dismutase (EC-SOD) targeted to type II cells protects postnatal newborn mouse lung development against hyperoxia by unknown mechanisms. Because alveolar development depends on timely proliferation of type II epithelium and differentiation to type I epithelium, we measured proliferation in bronchiolar and alveolar (surfactant protein C-positive) epithelium in air and 95% O2-exposed wild-type (WT) and TG hEC-SOD newborn mice at postnatal days 3, 5, and 7 (P3-P7), traversing the transition from saccular to alveolar stages. We found that TG hEC-SOD ameliorated the 95% O2-impaired bromodeoxyuridine uptake in alveolar and bronchiolar epithelium at P3, but not at P5 and P7, when overall epithelial proliferation rates were lower in air-exposed WT mice. Mouse EC-, CuZn-, and Mn-SOD expression were unaffected by hyperoxia or genotype. TG mice had less DNA damage than 95% O2-exposed WT mice at P3, measured by TdT-mediated dUTP nick end labeling (P < 0.05). Hyperoxia induced cell-cycle inhibitory protein p21cip/waf mRNA at P3, WT > TG, P = 0.06. 95% O2 impaired apical expression of type I cell alpha protein (T1alpha) in WT but not in TG mice at P3 and increased T1alpha in WT and TG mice at P7. Reducing the 95% O2-induced impairment of epithelial proliferation at a critical window of lung development was associated with protection against DNA damage and preservation of apical T1alpha expression at P3.
Figures





Similar articles
-
Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice.Am J Physiol Lung Cell Mol Physiol. 2009 Jul;297(1):L134-42. doi: 10.1152/ajplung.00112.2009. Epub 2009 May 1. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19411313 Free PMC article.
-
Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia.J Clin Invest. 1999 Apr;103(7):1055-66. doi: 10.1172/JCI3816. J Clin Invest. 1999. PMID: 10194479 Free PMC article.
-
Overexpression of extracellular superoxide dismutase has a protective role against hyperoxia-induced brain injury in neonatal mice.FEBS J. 2012 Mar;279(5):871-81. doi: 10.1111/j.1742-4658.2012.08478.x. Epub 2012 Feb 10. FEBS J. 2012. PMID: 22240000
-
Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung.Pediatr Res. 2011 May;69(5 Pt 1):371-7. doi: 10.1203/PDR.0b013e318211c917. Pediatr Res. 2011. PMID: 21270677 Free PMC article.
-
Superoxide dismutase and pulmonary oxygen toxicity: lessons from transgenic and knockout mice (Review).Int J Mol Med. 2001 Jan;7(1):13-9. doi: 10.3892/ijmm.7.1.13. Int J Mol Med. 2001. PMID: 11115602 Review.
Cited by
-
Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice.Antioxid Redox Signal. 2012 Nov 15;17(10):1376-92. doi: 10.1089/ars.2012.4572. Epub 2012 Jun 29. Antioxid Redox Signal. 2012. PMID: 22583151 Free PMC article.
-
Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10.J Biol Chem. 2010 Feb 26;285(9):5993-6002. doi: 10.1074/jbc.M109.075770. Epub 2010 Jan 6. J Biol Chem. 2010. PMID: 20053997 Free PMC article.
-
Pulmonary alveolar epithelial uptake of S-nitrosothiols is regulated by L-type amino acid transporter.Am J Physiol Lung Cell Mol Physiol. 2008 Jul;295(1):L38-43. doi: 10.1152/ajplung.00280.2007. Epub 2008 Apr 25. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18441097 Free PMC article.
-
Oxygen, gastrin-releasing Peptide, and pediatric lung disease: life in the balance.Front Pediatr. 2014 Jul 18;2:72. doi: 10.3389/fped.2014.00072. eCollection 2014. Front Pediatr. 2014. PMID: 25101250 Free PMC article. Review.
-
Lung-Specific Extracellular Superoxide Dismutase Improves Cognition of Adult Mice Exposed to Neonatal Hyperoxia.Front Med (Lausanne). 2018 Dec 10;5:334. doi: 10.3389/fmed.2018.00334. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30619855 Free PMC article.
References
-
- Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med. 2003;167:400–405. - PubMed
-
- Allred TF, Mercer RR, Thomas RF, Deng H, Auten RL. Brief 95% O2 exposure effects on surfactant protein and mRNA in rat alveolar and bronchiolar epithelium. Am J Physiol Lung Cell Mol Physiol. 1999;276:L999–L1009. - PubMed
-
- Asikainen TM, White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal. 2004;6:155–167. - PubMed
-
- Auten RL, Jr, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L336–L344. - PubMed
-
- Auten RL, Whorton MH, Nicholas Mason S. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol. 2002;26:391–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

