Chestnut as a food allergen: identification of major allergens
- PMID: 16100446
- PMCID: PMC2782150
- DOI: 10.3346/jkms.2005.20.4.573
Chestnut as a food allergen: identification of major allergens
Abstract
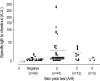
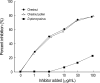
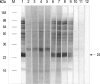
To evaluate the clinical significance of chestnut as a food allergen in Korea, skin prick test and ELISA were done in 1,738 patients with respiratory allergies. To identify the IgE binding components, IgE-immunoblotting, 2D IgE-immunoblotting and MALDITOF were performed. To observe the effects of digestive enzymes and a boiling treatment, simulated gastric fluid (SGF) and simulated intestinal fluids (SIF) were incubated with chestnut extracts, and IgE-immunoblotting were then repeated. Skin prick test revealed that 56 (3.2%) patients showed more than 2+ of allergen to histamine ratio to chestnut. Among the 21 IgE binding components, 9 bands were found in more than 50% of the sera tested and the 24 kDa protein had the highest binding intensity. The amino acid sequence of the 24 kDa protein (pI 6.3) had homology with legume protein of oak tree. SGF, SIF and boiling treatment were able to suppress the IgE binding components. In conclusion, chestnut ingestion was shown to induce IgE mediated responses with a 3.2% sensitization rate. Twenty one IgE binding components and one new allergen (the 24 kDa protein) were identified. Digestive enzymes and boiling treatment were able to decrease the allergenic potency.
Figures

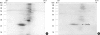
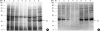
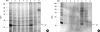
References
-
- Kim SH, Kang HR, Kim KM, Kim SS, Chang YS, Kim CW, Bahn JW, Kim YK, Cho SH, Park HS, Lee JM, Min KU, Hong CS, Kim NS, Kim YY. The sensitization rates of food allergens in a Korean population: a multi-center study (in Korean) J Asthma Allergy Clin Immunol. 2003;23:502–514.
-
- Yagame T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000;106:752–762. - PubMed
-
- Rico P, Crespo JF, Feliu A, Rodriguez J. Chestnut allergy: Beyond the latex-fruit syndrome. J Allergy Clin Immunol. 2004;113(Suppl 2)
-
- Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–1273. - PubMed
-
- Van Ree R. Clinical importance of non-specific lipid transfer proteins as food allergens. Biochem Soc Trans. 2002;30:910–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

