Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex
- PMID: 16100512
- PMCID: PMC1458033
- DOI: 10.1038/ncb1289
Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex
Abstract
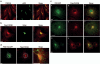
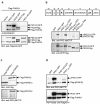
Protein kinase D (PKD) regulates the fission of vesicles originating from the trans-Golgi network. We show that phosphatidylinositol 4-kinase IIIbeta (PI4KIIIbeta) - a key player in the structure and function of the Golgi complex - is a physiological substrate of PKD. Of the three PKD isoforms, only PKD1 and PKD2 phosphorylated PI4KIIIbeta at a motif that is highly conserved from yeast to humans. PKD-mediated phosphorylation stimulated lipid kinase activity of PI4KIIIbeta and enhanced vesicular stomatitis virus G-protein transport to the plasma membrane. The identification of PI4KIIIbeta as one of the PKD substrates should help to reveal the molecular events that enable transport-carrier formation.
Figures


Comment in
-
Signalling for secretion.Nat Cell Biol. 2005 Sep;7(9):851-3. doi: 10.1038/ncb0905-851. Nat Cell Biol. 2005. PMID: 16136181 No abstract available.
References
-
- Liljedahl M, et al. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. - PubMed
-
- Godi A, et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulatessynthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. - PubMed
-
- Rykx A, et al. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

