The inhibitory effect of alendronate, a nitrogen-containing bisphosphonate on the PI3K-Akt-NFkappaB pathway in osteosarcoma cells
- PMID: 16100524
- PMCID: PMC1751194
- DOI: 10.1038/sj.bjp.0706373
The inhibitory effect of alendronate, a nitrogen-containing bisphosphonate on the PI3K-Akt-NFkappaB pathway in osteosarcoma cells
Abstract
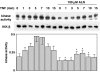
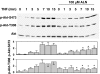
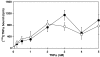
1 Bisphosphonates are inhibitors of tumor cell growth as well as of bone resorption by inducing cell apoptosis. However, little is known regarding the mechanisms by which the drug induces cell apoptosis. The aim of the present study was to determine the effect of alendronate, one of the nitrogen-containing bisphosphonates on the phoshoinositide 3-kinase (PI3K)-Akt-NFkappaB pathway, the major cell survival pathway. 2 The PI3K-Akt-NFkappaB pathway was activated in the osteosarcoma cell line MG-63 treated with tumor necrosis factor-alpha or insulin. Saos-2 was also used in some experiments. This was assessed by the production of phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P(3)), increased PI3K activity, phosphorylation of Akt at serine 473 and threonine 308, increase in activity of the inhibitor of nuclear factor kappaB (IkappaB) kinase (IKK) and finally phosphorylation of IkappaB and its subsequent degradation. 3 Pretreatment with alendronate at 100 microM for 24 h prior to the stimulation with tumor necrosis factor-alpha or insulin partially inhibited the IkappaB phosphorylation and degradation. These events were more clearly observed in the presence of inhibitors of proteasomes, which are responsible for the degradation of IkappaB. The drug also partially inhibited the activity of IKK, but almost fully inhibited the phosphorylation of Akt and the production of PtdIns(3,4,5)P(3). 4 The inhibitory effect of alendronate on IkappaB phosphorylation and degradation was not attenuated by the exogenous addition of geranylgeraniol to replenish the cytosolic isoprenyl lipid substrate. 5 The present findings demonstrate that alendronate inhibited the PI3K-Akt-NFkappaB cell survival pathway at the point of PI3K activation, thus indicating the presence of new targets of alendronate.
Figures

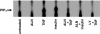

Similar articles
-
Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt.Int J Cancer. 2009 Dec 15;125(12):2863-70. doi: 10.1002/ijc.24748. Int J Cancer. 2009. PMID: 19609947 Free PMC article.
-
Id-1 activation of PI3K/Akt/NFkappaB signaling pathway and its significance in promoting survival of esophageal cancer cells.Carcinogenesis. 2007 Nov;28(11):2313-20. doi: 10.1093/carcin/bgm152. Epub 2007 Jul 16. Carcinogenesis. 2007. PMID: 17638919
-
Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells.J Biol Chem. 2003 Aug 1;278(31):28593-606. doi: 10.1074/jbc.M303445200. Epub 2003 May 27. J Biol Chem. 2003. PMID: 12771144
-
Osteopontin: it's role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression.IUBMB Life. 2005 Jun;57(6):441-7. doi: 10.1080/15216540500159424. IUBMB Life. 2005. PMID: 16012053 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Epidermal Growth Factor Reverses the Inhibitory Effects of the Bisphosphonate, Zoledronic Acid, on Human Oral Keratinocytes and Human Vascular Endothelial Cells In Vitro via the Epidermal Growth Factor Receptor (EGFR)/Akt/Phosphoinositide 3-Kinase (PI3K) Signaling Pathway.Med Sci Monit. 2019 Jan 24;25:700-710. doi: 10.12659/MSM.911579. Med Sci Monit. 2019. PMID: 30675875 Free PMC article.
-
Analysis of the molecular mechanism of osteosarcoma using a bioinformatics approach.Oncol Lett. 2016 Nov;12(5):3075-3080. doi: 10.3892/ol.2016.5060. Epub 2016 Aug 29. Oncol Lett. 2016. PMID: 27899966 Free PMC article.
-
Zoledronic acid reverses the epithelial-mesenchymal transition and inhibits self-renewal of breast cancer cells through inactivation of NF-κB.Mol Cancer Ther. 2013 Jul;12(7):1356-66. doi: 10.1158/1535-7163.MCT-12-0304. Epub 2013 Apr 25. Mol Cancer Ther. 2013. PMID: 23619300 Free PMC article.
-
CIP2A is overexpressed in osteosarcoma and regulates cell proliferation and invasion.Tumour Biol. 2014 Feb;35(2):1123-8. doi: 10.1007/s13277-013-1150-z. Epub 2013 Sep 8. Tumour Biol. 2014. PMID: 24014087
-
MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma.PLoS One. 2013;8(1):e53906. doi: 10.1371/journal.pone.0053906. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372675 Free PMC article.
References
-
- BENFORD H.L., FRITH J.C., AURIOLA S., MÖNKKÖNEN J., ROGERS M.J. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol. Pharmacol. 1999;56:131–140. - PubMed
-
- BEZZI M., HASMIM M., BIELER G., DORMOND O., RUEGG C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death. J. Biol. Chem. 2003;278:43603–43614. - PubMed
-
- BINDERMAN I., ADULT M., YAFFE A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. Periodontology. 2000;71:1236–1240. - PubMed
-
- CHENG Y.Y., HUANG L., LEE K.M., XU J.K., ZHENG M.H., KUMTA S.M. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif. Tissue Int. 2004;75:71–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

